Contribution of plasminogen activation towards the pathogenic potential of oral streptococci
- PMID: 21072208
- PMCID: PMC2972214
- DOI: 10.1371/journal.pone.0013826
Contribution of plasminogen activation towards the pathogenic potential of oral streptococci
Abstract
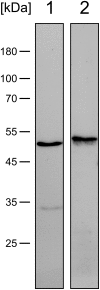
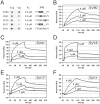
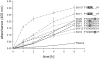
Oral streptococci are a heterogeneous group of human commensals, with a potential to cause serious infections. Activation of plasminogen has been shown to increase the virulence of typical human pathogenic streptococci such as S. pneumoniae. One important factor for plasminogen activation is the streptococcal α-enolase. Here we report that plasminogen activation is also common in oral streptococci species involved in clinical infection and that it depends on the action of human plasminogen activators. The ability to activate plasminogen did not require full conservation of the internal plasminogen binding sequence motif FYDKERKVY of α-enolase that was previously described as crucial for increased plasminogen binding, activation and virulence. Instead, experiments with recombinant α-enolase variants indicate that the naturally occurring variations do not impair plasminogen binding. In spite of these variations in the internal plasminogen binding motif oral streptococci showed similar activation of plasminogen. We conclude that the pathomechanism of plasminogen activation is conserved in oral streptococci that cause infections in human. This may contribute to their opportunistic pathogenic character that is unfurled in certain niches.
Conflict of interest statement
Figures



References
-
- Claridge JE, 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32:1511–1515. - PubMed
-
- Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: analysis of 19 cases. Clin Infect Dis. 1994;19:704–713. - PubMed
-
- Colombo AP, Haffajee AD, Dewhirst FE, Paster BJ, Smith CM, et al. Clinical and microbiological features of refractory periodontitis subjects. J Clin Periodontol. 1998;25:169–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

