The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+ -activated large-conductance K+ channels in endothelial cells
- PMID: 21072666
- PMCID: PMC3014879
- DOI: 10.1007/s00424-010-0898-x
The GPR55 agonist lysophosphatidylinositol acts as an intracellular messenger and bidirectionally modulates Ca2+ -activated large-conductance K+ channels in endothelial cells
Abstract
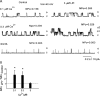
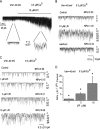
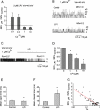
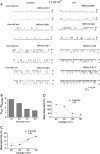
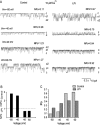
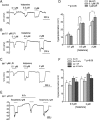
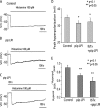
Lysophospholipids are known to serve as intra- and extracellular messengers affecting many physiological processes. Lysophosphatidylinositol (LPI), which is produced in endothelial cells, acts as an endogenous agonist of the orphan receptor, G protein-coupled receptor 55 (GPR55). Stimulation of GPR55 by LPI evokes an intracellular Ca(2+) rise in several cell types including endothelial cells. In this study, we investigated additional direct, receptor-independent effects of LPI on endothelial large-conductance Ca(2+) and voltage-gated potassium (BK(Ca)) channels. Electrophysiological experiments in the inside-out configuration revealed that LPI directly affects the BK(Ca) channel gating properties. This effect of LPI strictly depended on the presence of Ca(2+) and was concentration-dependent, reversible, and dual in nature. The modulating effects of LPI on endothelial BK(Ca) channels correlated with their initial open probability (Po): stimulation at low Po (<0.3) and inhibition at high Po levels (>0.3). In the whole-cell configuration, LPI in the pipette facilitated membrane hyperpolarization in response to low (0.1-2 μM) histamine concentrations. In contrast, LPI counteracted membrane hyperpolarization in response to supramaximal cell stimulation with histamine. These results highlight a novel receptor-independent and direct bidirectional modulation of BK(Ca) channels by LPI on endothelial cells. We conclude that LPI via this mechanism serves as an important modulator of endothelial electrical responses to cell stimulation.
Figures
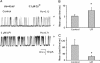
References
-
- Billah MM, Lapetina EG. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982;257:5196–5200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

