Inflammasomes: current understanding and open questions
- PMID: 21072676
- PMCID: PMC11114650
- DOI: 10.1007/s00018-010-0567-4
Inflammasomes: current understanding and open questions
Abstract
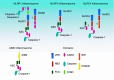
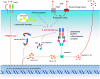
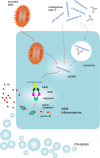
The innate immune system relies on its capability to detect invading microbes, tissue damage, or stress via evolutionarily conserved receptors. The nucleotide-binding domain leucine-rich repeat (NLR)-containing family of pattern recognition receptors includes several proteins that drive inflammation in response to a wide variety of molecular patterns. In particular, the NLRs that participate in the formation of a molecular scaffold termed the "inflammasome" have been intensively studied in past years. Inflammasome activation by multiple types of tissue damage or by pathogen-associated signatures results in the autocatalytic cleavage of caspase-1 and ultimately leads to the processing and thus secretion of pro-inflammatory cytokines, most importantly interleukin (IL)-1β and IL-18. Here, we review the current knowledge of mechanisms leading to the activation of inflammasomes. In particular, we focus on the controversial molecular mechanisms that regulate NLRP3 signaling and highlight recent advancements in DNA sensing by the inflammasome receptor AIM2.
Figures

References
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. - PubMed
-
- Faustin B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

