Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury
- PMID: 21073254
- PMCID: PMC3057520
- DOI: 10.3171/2010.10.JNS10925
Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury
Abstract
Object: Carbamylated erythropoietin (CEPO) is a modified erythropoietin molecule that does not affect hematocrit. In this study, the authors compared the efficacy of a single dose with a triple dose of CEPO treatment for traumatic brain injury (TBI) in rats.
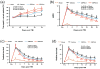
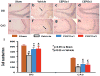
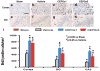
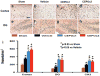
Methods: Traumatic brain injury was induced by controlled cortical impact over the left parietal cortex. Carbamylated erythropoietin (50 μg/kg) was administered intraperitoneally in rats with TBI at 6 hours (CEPO × 1) or at 6, 24, and 48 hours (CEPO × 3) postinjury. Neurological function was assessed using a modified neurological severity score and foot fault and Morris water maze tests. Animals were killed 35 days after injury, and brain sections were stained for immunohistochemical analysis to assess lesion volume, cell loss, cell proliferation, angiogenesis, and neurogenesis after CEPO treatment.
Results: Compared with the vehicle treatment, single treatment of CEPO (6 hours) significantly reduced lesion volume and hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, and significantly improved sensorimotor functional recovery and spatial learning in rats after TBI. Importantly, triple dosing of CEPO (6, 24, and 48 hours) further reduced lesion volume and improved functional recovery and neurogenesis compared with the CEPO × 1 group.
Conclusions: The authors' results indicate that CEPO has considerable therapeutic potential in TBI and related pathologies and furthermore that repeated dosing in the subacute phase might have important pharmacological relevance.
Conflict of interest statement
Figures



Similar articles
-
Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose.J Neurosurg. 2010 Sep;113(3):598-608. doi: 10.3171/2009.9.JNS09844. J Neurosurg. 2010. PMID: 19817538 Free PMC article.
-
Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats.J Neurosurg. 2011 Sep;115(3):550-60. doi: 10.3171/2011.3.JNS101721. Epub 2011 Apr 15. J Neurosurg. 2011. PMID: 21495821 Free PMC article.
-
Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit.Brain Res. 2009 Oct 19;1294:153-64. doi: 10.1016/j.brainres.2009.07.077. Epub 2009 Jul 30. Brain Res. 2009. PMID: 19646970 Free PMC article.
-
Carbamylated erythropoietin to treat neuronal injury: new development strategies.Expert Opin Investig Drugs. 2008 Aug;17(8):1175-86. doi: 10.1517/13543784.17.8.1175. Expert Opin Investig Drugs. 2008. PMID: 18616414 Review.
-
Angiogenesis, neurogenesis and brain recovery of function following injury.Curr Opin Investig Drugs. 2010 Mar;11(3):298-308. Curr Opin Investig Drugs. 2010. PMID: 20178043 Free PMC article. Review.
Cited by
-
Brain Phospholipid Precursors Administered Post-Injury Reduce Tissue Damage and Improve Neurological Outcome in Experimental Traumatic Brain Injury.J Neurotrauma. 2019 Jan 1;36(1):25-42. doi: 10.1089/neu.2017.5579. Epub 2018 Jul 25. J Neurotrauma. 2019. PMID: 29768974 Free PMC article.
-
A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury.CNS Drugs. 2012 Jul 1;26(7):613-36. doi: 10.2165/11634020-000000000-00000. CNS Drugs. 2012. PMID: 22668124 Review.
-
Erythropoietin Pathway: A Potential Target for the Treatment of Depression.Int J Mol Sci. 2016 May 6;17(5):677. doi: 10.3390/ijms17050677. Int J Mol Sci. 2016. PMID: 27164096 Free PMC article. Review.
-
Carbamylated erythropoietin attenuates cardiomyopathy via PI3K/Akt activation in rats with diabetic cardiomyopathy.Exp Ther Med. 2013 Aug;6(2):567-573. doi: 10.3892/etm.2013.1134. Epub 2013 May 31. Exp Ther Med. 2013. PMID: 24137228 Free PMC article.
-
Erythropoietin signaling increases neurogenesis and oligodendrogenesis of endogenous neural stem cells following spinal cord injury both in vivo and in vitro.Mol Med Rep. 2018 Jan;17(1):264-272. doi: 10.3892/mmr.2017.7873. Epub 2017 Oct 25. Mol Med Rep. 2018. PMID: 29115443 Free PMC article.
References
-
- Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, et al. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36:975–978. - PubMed
-
- Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. - PubMed
-
- Cerami A, Brines ML, Ghezzi P, Cerami CJ. Effects of epoetin alfa on the central nervous system. Semin Oncol. 2001;28:66–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

