Effect of LKB1 deficiency on mitochondrial content, fibre type and muscle performance in the mouse diaphragm
- PMID: 21073663
- PMCID: PMC3053411
- DOI: 10.1111/j.1748-1716.2010.02226.x
Effect of LKB1 deficiency on mitochondrial content, fibre type and muscle performance in the mouse diaphragm
Abstract
Aim: The liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) signalling pathway is a major regulator of skeletal muscle metabolic processes. During exercise, LKB1-mediated phosphorylation of AMPK leads to its activation, promoting mitochondrial biogenesis and glucose transport, among other effects. The roles of LKB1 and AMPK have not been fully characterized in the diaphragm.
Methods: Two methods of AMPK activation were used to characterize LKB1/AMPK signalling in diaphragms from muscle-specific LKB1 knockout (KO) and littermate control mice: (1) acute injection of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) and (2) 5-min direct electrical stimulation of the diaphragm. Diaphragms were excised 60 min post-AICAR injection and immediately after electrical stimulation.
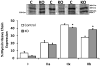
Results: AMPK phosphorylation increased with AICAR and electrical stimulation in control but not KO mice. Acetyl CoA carboxylase phosphorylation increased with AICAR in control but not KO mice, but increased in both genotypes with electrical stimulation. While the majority of mitochondrial protein levels were lower in KO diaphragms, uncoupling protein 3, complex I and cytochrome oxidase IV protein levels were not different between genotypes. KO diaphragms have a lower percentage of IIx fibres and an elevated percentage of IIb fibres when compared with control diaphragms. While in vitro peak force generation was similar between genotypes, KO diaphragms fatigued more quickly and had an impaired ability to recover.
Conclusion: LKB1 regulates AMPK phosphorylation, mitochondrial protein expression, fibre type distribution, as well as recovery of the diaphragm from fatigue.
Conflict of interest statement
Figures





References
-
- Bamford JA, Lopaschuk GD, MacLean IM, Reinhart ML, Dixon WT, Putman CT. Effects of chronic AICAR administration on the metabolic and contractile phenotypes of rat slow-and fast-twitch skeletal muscles. Can J Physiol Pharmacol. 2003;81:1072–82. - PubMed
-
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. American Journal of Physiology-Endocrinology and Metabolism. 2001;281:E1340–E1346. - PubMed
-
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–80. - PubMed
-
- Branvold DJ, Allred DR, Beckstead DJ, Kim HJ, Fillmore N, Condon BM, Brown JD, Sudweeks SN, Thomson DM, Winder WW. Thyroid hormone effects on LKB1, MO25, phospho-AMPK, phospho-CREB, and PGC-1alpha in rat muscle. J Appl Physiol. 2008;105:1218–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

