Absence of IFNγ expression induces neuronal degeneration in the spinal cord of adult mice
- PMID: 21073708
- PMCID: PMC2993684
- DOI: 10.1186/1742-2094-7-77
Absence of IFNγ expression induces neuronal degeneration in the spinal cord of adult mice
Abstract
Background: Interferon gamma (IFNγ) is a pro-inflammatory cytokine, which may be up-regulated after trauma to the peripheral or central nervous system. Such changes include reactive gliosis and synaptic plasticity that are considered important responses to the proper regenerative response after injury. Also, IFNγ is involved in the upregulation of the major histocompatibility complex class I (MHC class I), which has recently been shown to play an important role in the synaptic plasticity process following axotomy. There is also evidence that IFNγ may interfere in the differentiation and survival of neuronal cells. However, little is known about the effects of IFNγ absence on spinal cord neurons after injury.
Methods: We performed a unilateral sciatic nerve transection injury in C57BL/6J (wild type) and IFNγ-KO (mutant) mice and studied motoneuron morphology using light and electron microscopy. One week after the lesion, mice from both strains were sacrificed and had their lumbar spinal cords processed for histochemistry (n = 5 each group) and transmission electron microscopy (TEM, n = 5 each group). Spinal cord sections from non-lesioned animals were also used to investigate neuronal survival and the presence of apoptosis with TUNEL and immunohistochemistry.
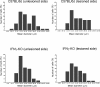
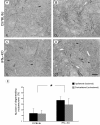
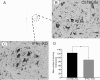
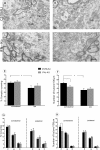
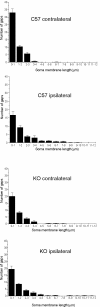
Results: We find that presumed motoneurons in the lower lumbar ventral horn exhibited a smaller soma size in the IFNγ-KO series, regardless of nerve lesion. In plastic embedded sections stained with toluidine blue, the IFNγ-KO mice demonstrated a greater proportion of degenerating neurons in the ventral horn when compared to the control series (p < 0.05). Apoptotic death is suggested based on TUNEL and caspase 3 immunostaining. A sciatic nerve axotomy did not further aggravate the neuronal loss. The cellular changes were supported by electron microscopy, which demonstrated ventral horn neurons exhibiting intracellular vacuoles as well as degenerating nuclei and cytoplasm in the IFNγ-KO mice. Adjacent glial cells showed features suggestive of phagocytosis. Additional ultrastructural studies showed a decreased number of pre-synaptic terminals apposing to motoneurons in mutant mice. Nevertheless, no statistical difference regarding the input covering could be detected among the studied strains.
Conclusion: Altogether, these results suggest that IFNγ may be neuroprotective and its absence results in neuronal death, which is not further increased by peripheral axotomy.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

