The membrane-spanning domain of gp41 plays a critical role in intracellular trafficking of the HIV envelope protein
- PMID: 21073746
- PMCID: PMC2994783
- DOI: 10.1186/1742-4690-7-95
The membrane-spanning domain of gp41 plays a critical role in intracellular trafficking of the HIV envelope protein
Abstract
Background: The sequences of membrane-spanning domains (MSDs) on the gp41 subunit are highly conserved among many isolates of HIV-1. The GXXXG motif, a potential helix-helix interaction motif, and an arginine residue (rare in hydrophobic MSDs) are especially well conserved. These two conserved elements are expected to locate on the opposite sides of the MSD, if the MSD takes a α-helical secondary structure. A scanning alanine-insertion mutagenesis was performed to elucidate the structure-function relationship of gp41 MSD.
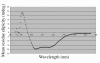
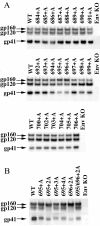
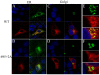
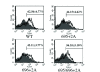
Results: A circular dichroism analysis of a synthetic gp41 MSD peptide determined that the secondary structure of the gp41 MSD was α-helical. We then performed a scanning alanine-insertion mutagenesis of the entire gp41 MSD, progressively shifting the relative positions of MSD segments around the helix axis. Altering the position of Gly694, the last residue of the GXXXG motif, relative to Arg696 (the number indicates the position of the amino acid residues in HXB2 Env) around the axis resulted in defective fusion. These mutants showed impaired processing of the gp160 precursor into gp120 and gp41. Furthermore, these Env mutants manifested inefficient intracellular transport in the endoplasmic reticulum and Golgi regions. Indeed, a transplantation of the gp41 MSD portion into the transmembrane domain of another membrane protein, Tac, altered its intracellular distribution. Our data suggest that the intact MSD α-helix is critical in the intracellular trafficking of HIV-1 Env.
Conclusions: The relative position between the highly conserved GXXXG motif and an arginine residue around the gp41 MSD α-helix is critical for intracellular trafficking of HIV-1 Env. The gp41 MSD region not only modulates membrane fusion but also controls biosynthesis of HIV-1 Env.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

