Structural insight into the expanded PCB-degrading abilities of a biphenyl dioxygenase obtained by directed evolution
- PMID: 21073881
- PMCID: PMC3102011
- DOI: 10.1016/j.jmb.2010.11.009
Structural insight into the expanded PCB-degrading abilities of a biphenyl dioxygenase obtained by directed evolution
Abstract
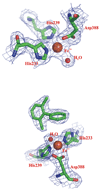
The biphenyl dioxygenase of Burkholderia xenovorans LB400 is a multicomponent Rieske-type oxygenase that catalyzes the dihydroxylation of biphenyl and many polychlorinated biphenyls (PCBs). The structural bases for the substrate specificity of the enzyme's oxygenase component (BphAE(LB400)) are largely unknown. BphAE(p4), a variant previously obtained through directed evolution, transforms several chlorobiphenyls, including 2,6-dichlorobiphenyl, more efficiently than BphAE(LB400), yet differs from the parent oxygenase at only two positions: T335A/F336M. Here, we compare the structures of BphAE(LB400) and BphAE(p4) and examine the biochemical properties of two BphAE(LB400) variants with single substitutions, T335A or F336M. Our data show that residue 336 contacts the biphenyl and influences the regiospecificity of the reaction, but does not enhance the enzyme's reactivity toward 2,6-dichlorobiphenyl. By contrast, residue 335 does not contact biphenyl but contributes significantly to expansion of the enzyme's substrate range. Crystal structures indicate that Thr335 imposes constraints through hydrogen bonds and nonbonded contacts to the segment from Val320 to Gln322. These contacts are lost when Thr is replaced by Ala, relieving intramolecular constraints and allowing for significant movement of this segment during binding of 2,6-dichlorobiphenyl, which increases the space available to accommodate the doubly ortho-chlorinated congener 2,6-dichlorobiphenyl. This study provides important insight about how Rieske-type oxygenases can expand substrate range through mutations that increase the plasticity and/or mobility of protein segments lining the catalytic cavity.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Engineering Burkholderia xenovorans LB400 BphA through Site-Directed Mutagenesis at Position 283.Appl Environ Microbiol. 2020 Sep 17;86(19):e01040-20. doi: 10.1128/AEM.01040-20. Print 2020 Sep 17. Appl Environ Microbiol. 2020. PMID: 32709719 Free PMC article.
-
Characterization of biphenyl dioxygenase of Pandoraea pnomenusa B-356 as a potent polychlorinated biphenyl-degrading enzyme.J Bacteriol. 2007 Aug;189(15):5705-15. doi: 10.1128/JB.01476-06. Epub 2007 May 25. J Bacteriol. 2007. PMID: 17526697 Free PMC article.
-
Retuning Rieske-type oxygenases to expand substrate range.J Biol Chem. 2011 Aug 5;286(31):27612-21. doi: 10.1074/jbc.M111.255174. Epub 2011 Jun 8. J Biol Chem. 2011. PMID: 21653696 Free PMC article.
-
Rieske business: structure-function of Rieske non-heme oxygenases.Biochem Biophys Res Commun. 2005 Dec 9;338(1):175-90. doi: 10.1016/j.bbrc.2005.08.222. Epub 2005 Sep 8. Biochem Biophys Res Commun. 2005. PMID: 16168954 Review.
-
A profile of ring-hydroxylating oxygenases that degrade aromatic pollutants.Rev Environ Contam Toxicol. 2010;206:65-94. doi: 10.1007/978-1-4419-6260-7_4. Rev Environ Contam Toxicol. 2010. PMID: 20652669 Review.
Cited by
-
Biphenyl 2,3-Dioxygenase in Pseudomonas alcaliphila JAB1 Is Both Induced by Phenolics and Monoterpenes and Involved in Their Transformation.Front Microbiol. 2021 Apr 30;12:657311. doi: 10.3389/fmicb.2021.657311. eCollection 2021. Front Microbiol. 2021. PMID: 33995321 Free PMC article.
-
The α- and β-Subunit Boundary at the Stem of the Mushroom-Like α3β3-Type Oxygenase Component of Rieske Non-Heme Iron Oxygenases Is the Rieske-Type Ferredoxin-Binding Site.Appl Environ Microbiol. 2022 Aug 9;88(15):e0083522. doi: 10.1128/aem.00835-22. Epub 2022 Jul 13. Appl Environ Microbiol. 2022. PMID: 35862661 Free PMC article.
-
Structural Basis of the Enhanced Pollutant-Degrading Capabilities of an Engineered Biphenyl Dioxygenase.J Bacteriol. 2016 Apr 28;198(10):1499-512. doi: 10.1128/JB.00952-15. Print 2016 May 15. J Bacteriol. 2016. PMID: 26953337 Free PMC article.
-
Engineering Burkholderia xenovorans LB400 BphA through Site-Directed Mutagenesis at Position 283.Appl Environ Microbiol. 2020 Sep 17;86(19):e01040-20. doi: 10.1128/AEM.01040-20. Print 2020 Sep 17. Appl Environ Microbiol. 2020. PMID: 32709719 Free PMC article.
-
Burkholderia denitrificans sp. nov., isolated from the soil of Dokdo Island, Korea.J Microbiol. 2012 Oct;50(5):855-9. doi: 10.1007/s12275-012-1554-2. Epub 2012 Nov 4. J Microbiol. 2012. PMID: 23124756
References
-
- Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters--what we have learned from Yusho disease. Environ Res. 2001;86:2–11. - PubMed
-
- Safe SH. Polychlorinated biphenyls (PCBs) - Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. - PubMed
-
- Misawa N, Nakamura R, Kagiyama Y, Ikenaga H, Furukawa K, Shindo K. Synthesis of vicinal diols from various arenes with a heterocyclic, amino or carboxyl group by using recombinant Escherichia coli cells expressing evolved biphenyl dioxygenase and dihydrodiol dehydrogenase genes. Tetrahedron. 2005;61:195–204.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

