A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells
- PMID: 21074045
- PMCID: PMC3003590
- DOI: 10.1016/j.cell.2010.10.016
A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells
Abstract
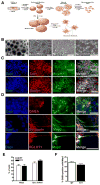
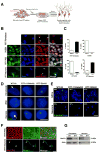
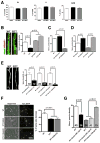
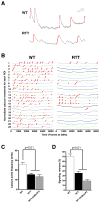
Autism spectrum disorders (ASD) are complex neurodevelopmental diseases in which different combinations of genetic mutations may contribute to the phenotype. Using Rett syndrome (RTT) as an ASD genetic model, we developed a culture system using induced pluripotent stem cells (iPSCs) from RTT patients' fibroblasts. RTT patients' iPSCs are able to undergo X-inactivation and generate functional neurons. Neurons derived from RTT-iPSCs had fewer synapses, reduced spine density, smaller soma size, altered calcium signaling and electrophysiological defects when compared to controls. Our data uncovered early alterations in developing human RTT neurons. Finally, we used RTT neurons to test the effects of drugs in rescuing synaptic defects. Our data provide evidence of an unexplored developmental window, before disease onset, in RTT syndrome where potential therapies could be successfully employed. Our model recapitulates early stages of a human neurodevelopmental disease and represents a promising cellular tool for drug screening, diagnosis and personalized treatment.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Modeling Rett syndrome with stem cells.Cell. 2010 Nov 12;143(4):499-500. doi: 10.1016/j.cell.2010.10.037. Cell. 2010. PMID: 21074040 Free PMC article.
References
-
- Prevalence of autism spectrum disorders--autism and developmental disabilities monitoring network, six sites, United States. MMWR Surveill Summ. 2000;56:1–11. - PubMed
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

