Developmental control of late replication and S phase length
- PMID: 21074439
- PMCID: PMC3108027
- DOI: 10.1016/j.cub.2010.10.021
Developmental control of late replication and S phase length
Abstract
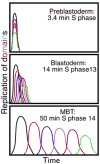
Background: Fast, early embryonic cell cycles have correspondingly fast S phases. In early Drosophila embryos, forks starting from closely spaced origins replicate the whole genome in 3.4 min, ten times faster than in embryonic cycle 14 and a hundred times faster than in a wing disc. It is not known how S phase duration is regulated. Here we examined prolongation of embryonic S phases, its coupling to development, and its relationship to the appearance of heterochromatin.
Results: Imaging of fluorescent nucleotide incorporation and GFP-PCNA gave exquisite time resolution of S phase events. In the early S phases, satellite sequences replicated rapidly despite a compact chromatin structure. In S phases 11-13, a delay in satellite replication emerged in sync with modest and progressive prolongation of S phase. In S phase 14, major and distinct delays ordered the replication of satellites into a sequence that occupied much of S phase. This onset of late replication required transcription. Satellites only accumulated abundant heterochromatin protein 1 (HP1) after replicating in S phase 14. By cycle 15, satellites clustered in a compact HP1-positive mass, but replication occurred at decondensed foci at the surface of this mass.
Conclusions: The slowing of S phase is an active process, not a titration of maternal replication machinery. Most sequences continue to replicate rapidly in successive cycles, but increasing delays in the replication of satellite sequences extend S phase. Although called constitutively heterochromatic, satellites acquire the distinctive features of heterochromatin, compaction, late replication, HP1 binding, and aggregation at the chromocenter, in successive steps coordinated with developmental progress.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures







Comment in
-
Heterochromatin replication: better late than ever.Curr Biol. 2010 Dec 7;20(23):R1018-20. doi: 10.1016/j.cub.2010.11.003. Curr Biol. 2010. PMID: 21145016
References
-
- Brown SW. Heterochromatin. Science. 1966;151:417–425. - PubMed
-
- Lucchesi JC, Kelly WG, Panning B. Chromatin remodeling in dosage compensation. Annu Rev Genet. 2005;39:615–651. - PubMed
-
- Dalle Nogare DE, Pauerstein PT, Lane ME. G2 acquisition by transcription-independent mechanism at the zebrafish midblastula transition. Dev Biol. 2009;326:131–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

