Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice
- PMID: 21074540
- PMCID: PMC3397477
- DOI: 10.1016/j.yjmcc.2010.11.002
Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice
Abstract
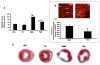
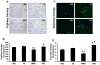
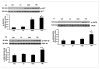
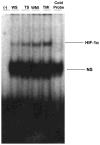
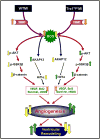
Oxidative stress plays a crucial role in disruption of neovascularization by alterations in thioredoxin 1 (Trx1) expression and its interaction with other proteins after myocardial infarction (MI). We previously showed that Trx1 has angiogenic properties, but the possible therapeutic significance of overexpressing Trx1 in chronic MI has not been elucidated. Therefore, we explored the angiogenic and cardioprotective potential of Trx1 in an in vivo MI model using transgenic mice overexpressing Trx1. Wild-type (W) and Trx1 transgenic (Trx1(Tg/+)) mice were randomized into W sham (WS), Trx1(Tg/+) sham (TS), WMI, and TMI. MI was induced by permanent occlusion of LAD coronary artery. Hearts from mice overexpressing Trx1 exhibited reduced fibrosis and oxidative stress and attenuated cardiomyocyte apoptosis along with increased vessel formation compared to WMI. We found significant inhibition of Trx1 regulating proteins, TXNIP and AKAP 12, and increased p-Akt, p-eNOS, p-GSK-3β, HIF-1α, β-catenin, VEGF, Bcl-2, and survivin expression in TMI compared to WMI. Echocardiography performed 30days after MI revealed significant improvement in myocardial functions in TMI compared to WMI. Our study identifies a potential role for Trx1 overexpression and its association with its regulatory proteins TXNIP, AKAP12, and subsequent activation of Akt/GSK-3β/β-catenin/HIF-1α-mediated VEGF and eNOS expression in inducing angiogenesis and reduced ventricular remodeling. Hence, Trx1 and other proteins identified in our study may prove to be potential therapeutic targets in the treatment of ischemic heart disease.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


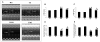
References
-
- Molin D, Post MJ. Therapeutic angiogenesis in the heart: protect and serve. Curr Opin Pharmacol. 2007;7:158–63. - PubMed
-
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–91. - PubMed
-
- Billiet L, Rouis M. Thioredoxin-1 is a novel and attractive therapeutic approach for various diseases including cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets. 2008;8:293–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

