Nonesterified cholesterol content of lysosomes modulates susceptibility to oxidant-induced permeabilization
- PMID: 21074609
- PMCID: PMC3018561
- DOI: 10.1016/j.freeradbiomed.2010.11.006
Nonesterified cholesterol content of lysosomes modulates susceptibility to oxidant-induced permeabilization
Abstract
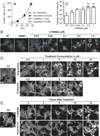
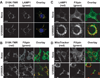
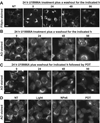
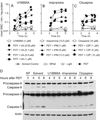
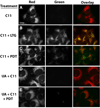
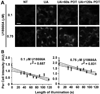
Reactive oxygen species (ROS) can induce lysosomal membrane permeabilization (LMP). Photoirradiation of murine hepatoma 1c1c7 cultures preloaded with the photosensitizer NPe6 generates singlet oxygen within acidic organelles and causes LMP and the activation of procaspases. Treatment with the cationic amphiphilic drugs (CADs) U18666A, imipramine, and clozapine stimulated the accumulation of filipin-stainable nonesterified cholesterol/sterols in late endosomes/lysosomes, but not in mitochondria. Concentration-response studies demonstrated an inverse relationship between lysosomal nonesterified cholesterol/sterol contents and susceptibility to NPe6 photoirradiation-induced intracellular membrane oxidation, LMP, and activation of procaspase-9 and -3. Similarly, the kinetics of restoration of NPe6 photoirradiation-induced LMP paralleled the losses of lysosomal cholesterol that occurred upon replating U18666A-treated cultures in CAD-free medium. Consistent with the oxidation of lysosomal cholesterol, filipin staining in U18666A-treated cultures progressively decreased with increasing photoirradiating light dose. U18666A also suppressed the induction of LMP and procaspase activation by exogenously added hydrogen peroxide. However, neither U18666A nor imipramine suppressed the induction of apoptosis by agents that did not directly induce LMP. These studies indicate that lysosomal nonesterified cholesterol/sterol content modulates susceptibility to ROS-induced LMP and possibly does so by being an alternative target for oxidants and lowering the probability of damage to other lysosomal membrane lipids and/or proteins.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–2890. - PubMed
-
- Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta. 2009;1793:746–754. - PubMed
-
- Dare E, Li W, Zhivotorsky B, Yuan S, Ceccatelli S. Methyl mercury and H2O2 provoke lysosomal damage in human astrocytoma D384 cells followed by apoptosis. Free Radic. Biol. Med. 2001;30:1347–1356. - PubMed
-
- Kurtz T, Gustafsson B, Brunk UT. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006;273:3106–3117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

