Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro
- PMID: 21074619
- PMCID: PMC3293703
- DOI: 10.1016/j.jsb.2010.11.006
Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro
Abstract
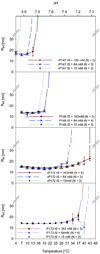
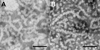
The self-assembly of the predominant extracellular enamel matrix protein amelogenin plays an essential role in regulating the growth and organization of enamel mineral during early stages of dental enamel formation. The present study describes the effect of the phosphorylation of a single site on the full-length native porcine amelogenin P173 on self-assembly and on the regulation of spontaneous calcium phosphate formation in vitro. Studies were also conducted using recombinant non-phosphorylated (rP172) porcine amelogenin, along with the most abundant amelogenin cleavage product (P148) and its recombinant form (rP147). Amelogenin self-assembly was assessed using dynamic light scattering (DLS) and transmission electron microscopy (TEM). Using these approaches, we have shown that self-assembly of each amelogenin is very sensitive to pH and appears to be affected by both hydrophilic and hydrophobic interactions. Furthermore, our results suggest that the phosphorylation of the full-length porcine amelogenin P173 has a small but potentially important effect on its higher-order self-assembly into chain-like structures under physiological conditions of pH, temperature, and ionic strength. Although phosphorylation has a subtle effect on the higher-order assembly of full-length amelogenin, native phosphorylated P173 was found to stabilize amorphous calcium phosphate for extended periods of time, in sharp contrast to previous findings using non-phosphorylated rP172. The biological relevance of these findings is discussed.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Regulation of calcium phosphate formation by amelogenins under physiological conditions.Eur J Oral Sci. 2011 Dec;119 Suppl 1(Suppl 1):103-11. doi: 10.1111/j.1600-0722.2011.00911.x. Eur J Oral Sci. 2011. PMID: 22243235 Free PMC article.
-
Role of 20-kDa amelogenin (P148) phosphorylation in calcium phosphate formation in vitro.J Biol Chem. 2009 Jul 10;284(28):18972-9. doi: 10.1074/jbc.M109.020370. Epub 2009 May 14. J Biol Chem. 2009. PMID: 19443653 Free PMC article.
-
MMP20 Proteolysis of Native Amelogenin Regulates Mineralization In Vitro.J Dent Res. 2016 Dec;95(13):1511-1517. doi: 10.1177/0022034516662814. Epub 2016 Aug 24. J Dent Res. 2016. PMID: 27558264 Free PMC article.
-
Assembly of amelogenin proteolytic products and control of octacalcium phosphate crystal morphology.Connect Tissue Res. 2003;44 Suppl 1:58-64. Connect Tissue Res. 2003. PMID: 12952175 Review.
-
Mechanisms of Enamel Mineralization Guided by Amelogenin Nanoribbons.J Dent Res. 2021 Dec;100(13):1434-1443. doi: 10.1177/00220345211012925. Epub 2021 May 19. J Dent Res. 2021. PMID: 34009057 Free PMC article. Review.
Cited by
-
Remineralization Efficacy of an Amelogenin-Based Synthetic Peptide on Carious Lesions.Front Physiol. 2018 Jul 5;9:842. doi: 10.3389/fphys.2018.00842. eCollection 2018. Front Physiol. 2018. PMID: 30026702 Free PMC article.
-
Regulation of Hydroxyapatite Nucleation In Vitro through Ameloblastin-Amelogenin Interactions.ACS Biomater Sci Eng. 2023 Apr 10;9(4):1834-1842. doi: 10.1021/acsbiomaterials.1c01113. Epub 2022 Jan 24. ACS Biomater Sci Eng. 2023. PMID: 35068157 Free PMC article.
-
Leucine-rich amelogenin peptides regulate mineralization in vitro.J Dent Res. 2011 Sep;90(9):1091-7. doi: 10.1177/0022034511411301. Epub 2011 Jun 7. J Dent Res. 2011. PMID: 21653221 Free PMC article.
-
Entering the era of nanoscience: time to be so small.J Biomed Nanotechnol. 2013 Sep;9(9):1441-70. doi: 10.1166/jbn.2013.1642. J Biomed Nanotechnol. 2013. PMID: 23980495 Free PMC article. Review.
-
Structural changes in amelogenin upon self-assembly and mineral interactions.J Dent Res. 2012 Oct;91(10):967-72. doi: 10.1177/0022034512457371. Epub 2012 Aug 28. J Dent Res. 2012. PMID: 22933608 Free PMC article.
References
-
- Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, Sreenath T, Wright JT, Decker S, Piddington R, Harrison G, Kulkarni AB. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J. Biol. Chem. 2001;276:31871–31875. - PubMed
-
- Arsenault AL, Robinson BW. The dentino-enamel junction: a structural and microanalytical study of early mineralization. Calcif. Tiss. Int. 1989;45:111–121. - PubMed
-
- Smith CE. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 1998;9:128–161. - PubMed
-
- Uchida T, Tanabe T, Fukae M, Shimizu M, Yamada M, Miake K, Kobayashi S. Immunochemical and immunohistochemical studies, using antisera against porcine 25 kDa amelogenin, 89 kDa enamelin and the 13–17 kDa nonamelogenins, on immature enamel of the pig and rat. Histochem. 1991;96:129–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

