Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China
- PMID: 21075054
- PMCID: PMC5716831
- DOI: 10.1016/S1470-2045(10)70256-4
Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China
Erratum in
- Lancet Oncol. 2011 Jan;12(1):11
Abstract
Background: Controversy remains over whether high-risk human papillomavirus (HPV) DNA testing should be used as a primary screen for cervical cancer. The aims of our study were to assess whether HPV DNA testing could be applied to cervical-cancer screening programmes in China, as well as other similar developing countries.
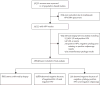
Methods: We did a pooled analysis of population-based cervical cancer screening studies done in mainland China from 1999 to 2008 with concurrent HPV DNA testing (Hybrid Capture 2 assay; Qiagen, Gaithersburg, MD, USA), liquid-based cytology (LBC), and visual inspection with acetic acid (VIA). Eligible women were sexually active, not pregnant, had an intact uterus, and had no history of cervical intraepithelial neoplasia (CIN), cervical cancer, or pelvic irradiation. All women positive for any test were referred for colposcopy and biopsy. Cervical lesions were diagnosed by directed or random biopsy. We assessed the diagnostic accuracy of HPV DNA testing for the detection of CIN grade 3 or greater.
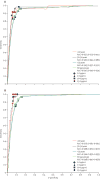
Findings: 30,371 women from 17 cross-sectional, population-based studies in various parts of China were screened. 1523 women were subsequently excluded because of inadequate HPV DNA specimens or they did not have a biopsy taken, which included women with atypical squamous cells of undetermined significance; low-grade squamous intraepithelial lesion or worse; positive HPV, negative cytology, and missing or positive colposcopy results; and unsatisfactory cytology results. HPV DNA testing had a higher sensitivity of 97·5% (95% CI 95·7-98·7) for detection of CIN grade 3 or worse, and a lower specificity of 85·1% (82·3-87·9), compared with cytology (sensitivity 87·9% [95% CI 84·7-90·7], specificity 94·7% [93·5-96·0]) and VIA (54·6% [48·0-61·2], 89·9% [86·8-93·0]). Sensitivity did not vary by study or age (<35 years, 35-49 years, ≥50 years); however, specificity did vary with age (p<0·0001) and was highest in women younger than 35 years (89·4%; 95% CI 86·1-91·5). An increase in the positive cutoff point from the manufacturer recommended 1 pg/mL to 2 pg/mL led to a decrease in the overall HPV DNA positivity from 16·3% to 13·9% (p<0·0001), which could result in a decrease in referral rates, although sensitivity was slightly lower (97·5% to 95·2%). An increase in the cutoff point to 10 pg/mL in women younger than 35 years maintained a high sensitivity 97·7% (95% CI 87·7-99·9) and increased specificity to 93·5% (95% CI 91·9-94·6).
Interpretation: HPV DNA testing is highly sensitive and moderately specific for CIN grade 3 or worse, with consistent results across study sites and age groups-including women younger than 35 years. A rise in the cutoff point might be beneficial for future screening programmes in China, especially when screening women younger than 35 years.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures

Comment in
-
HPV-based cervical-cancer screening in China.Lancet Oncol. 2010 Dec;11(12):1112-3. doi: 10.1016/S1470-2045(10)70262-X. Epub 2010 Nov 11. Lancet Oncol. 2010. PMID: 21075053 No abstract available.
References
-
- Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006;24(suppl 3):S3/11–25. - PubMed
-
- International Agency for Research on Cancer. IARC handbooks of cancer prevention: cervix cancer screening. Vol. 10. Lyon: IARC Press; 2005. Chapter 2: screening tests; pp. 59–115.
-
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans, vol 90: human papillomaviruses. Lyon: IARC Press; 2007.
-
- Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11:249–57. - PubMed
-
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10:672–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

