Evidence for prenylation-dependent targeting of a Ykt6 SNARE in Plasmodium falciparum
- PMID: 21075148
- PMCID: PMC3030980
- DOI: 10.1016/j.molbiopara.2010.11.007
Evidence for prenylation-dependent targeting of a Ykt6 SNARE in Plasmodium falciparum
Abstract
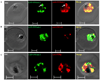
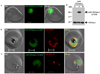
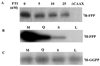
Ykt6 proteins are the most versatile fusogens in eukaryotic cells, and the only SNAREs that can be both prenylated and acylated at a C-terminal CAAX motif. Unlike yeast and mammalian cells where a single Ykt6 gene is expressed, the Plasmodium falciparum genome encodes two Ykt6 proteins. We have investigated the expression and prenylation of the Ykt6 orthologue, PfYkt6.1 in intra-erythrocytic stages of P. falciparum. PfYkt6.1 localized to the parasite Golgi and other unidentified cytoplasmic compartments, and was partly cytosolic (∼50% in early trophozoites). The membrane-association of PfYkt6.1 was dependent on the presence of a conserved C-terminal CAAX motif (CCSIM). By expressing full-length and mutant proteins in Escherichia coli, we have shown that PfYkt6.1 indeed serves as substrate for prenylation by P. falciparum farnesyltransferases. Surprisingly, PfYkt6.1 could also be geranylgeranylated by parasite extracts independent of the C-terminal amino acid residue. Deletion of the CAAX motif inhibited both farnesylation and geranylgeranylation activities. Additionally, the PfYkt6.1 heptapeptide KQCCSIM, corresponding to the C-terminal CAAX sequence, inhibited the parasite farnesyltransferase activity with an IC(50) of 1 μM. Our findings underscore the importance of CAAX motif-derived peptidomimetics for antimalarial drug development.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Double prenylation of SNARE protein Ykt6 is required for lysosomal hydrolase trafficking.J Biochem. 2021 Apr 18;169(3):363-370. doi: 10.1093/jb/mvaa111. J Biochem. 2021. PMID: 33035318
-
The Prenylated Proteome of Plasmodium falciparum Reveals Pathogen-specific Prenylation Activity and Drug Mechanism-of-action.Mol Cell Proteomics. 2017 Apr;16(4 suppl 1):S54-S64. doi: 10.1074/mcp.M116.064550. Epub 2016 Dec 31. Mol Cell Proteomics. 2017. PMID: 28040698 Free PMC article.
-
Use of Biotin-Labeled Geranyl Pyrophosphate for Analysis of Ykt6 Geranylgeranylation.Methods Mol Biol. 2025;2887:93-102. doi: 10.1007/978-1-0716-4314-3_6. Methods Mol Biol. 2025. PMID: 39806148
-
Enzymology and biology of CaaX protein prenylation.Recent Prog Horm Res. 1999;54:315-42; discussion 342-3. Recent Prog Horm Res. 1999. PMID: 10548882 Review.
-
Isoprenoid biosynthesis in the erythrocytic stages of Plasmodium falciparum.Mem Inst Oswaldo Cruz. 2011 Aug;106 Suppl 1:134-41. doi: 10.1590/s0074-02762011000900018. Mem Inst Oswaldo Cruz. 2011. PMID: 21881768 Review.
Cited by
-
Cryptic organelle homology in apicomplexan parasites: insights from evolutionary cell biology.Curr Opin Microbiol. 2013 Aug;16(4):424-31. doi: 10.1016/j.mib.2013.07.015. Epub 2013 Aug 8. Curr Opin Microbiol. 2013. PMID: 23932202 Free PMC article.
-
Protein Sorting in Plasmodium Falciparum.Life (Basel). 2021 Sep 9;11(9):937. doi: 10.3390/life11090937. Life (Basel). 2021. PMID: 34575086 Free PMC article. Review.
-
Global proteomic analysis of prenylated proteins in Plasmodium falciparum using an alkyne-modified isoprenoid analogue.Sci Rep. 2016 Dec 7;6:38615. doi: 10.1038/srep38615. Sci Rep. 2016. PMID: 27924931 Free PMC article.
-
Functional screening reveals Toxoplasma prenylated proteins required for endocytic trafficking and rhoptry protein sorting.mBio. 2023 Aug 31;14(4):e0130923. doi: 10.1128/mbio.01309-23. Epub 2023 Aug 7. mBio. 2023. PMID: 37548452 Free PMC article.
-
ELAPOR1 is a copper-dependent tethering factor driving proacrosomal vesicle fusion during acrosome biogenesis.Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2501302122. doi: 10.1073/pnas.2501302122. Epub 2025 Jul 30. Proc Natl Acad Sci U S A. 2025. PMID: 40737321
References
-
- Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:120–144. - PubMed
-
- Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. - PubMed
-
- Pelham HR. SNAREs and the specificity of membrane fusion. Trends Cell Biol. 2001;11:99–101. - PubMed
-
- Borgese N, Brambillasca S, Soffientini P, Yabal M, Makarow M. Biogenesis of tail-anchored proteins. Biochem Soc Trans. 2003;31:1238–1242. - PubMed
-
- Steel GJ, Brownsword J, Stirling CJ. Tail-anchored protein insertion into yeast ER requires a novel posttranslational mechanism which is independent of the SEC machinery. Biochemistry. 2002;41:11914–11920. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

