Novel mechanism of increased Ca2+ release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors
- PMID: 21075175
- PMCID: PMC3038464
- DOI: 10.1016/j.neuroscience.2010.11.010
Novel mechanism of increased Ca2+ release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors
Abstract
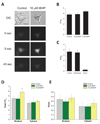
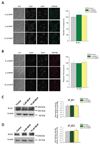
Dysregulation of Ca(2+) signaling following oxidative stress is an important pathophysiological mechanism of many chronic neurodegenerative disorders, including Alzheimer's disease, age-related macular degeneration, glaucomatous and diabetic retinopathies. However, the underlying mechanisms of disturbed intracellular Ca(2+) signaling remain largely unknown. We here describe a novel mechanism for increased intracellular Ca(2+) release following oxidative stress in a neuronal cell line. Using an experimental approach that included quantitative polymerase chain reaction, quantitative immunoblotting, microfluorimetry and the optical imaging of intracellular Ca(2+) release, we show that sub-lethal tert-butyl hydroperoxide-mediated oxidative stress result in a selective up-regulation of type-2 inositol-1,4,5,-trisphophate receptors. This oxidative stress mediated change was detected both at the transcriptional and translational level and functionally resulted in increased Ca(2+) release into the nucleoplasm from the membranes of the nuclear envelope at a given receptor-specific stimulus. Our data describe a novel source of Ca(2+) dysregulation induced by oxidative stress with potential relevance for differential subcellular Ca(2+) signaling specifically within the nucleus and the development of novel neuroprotective strategies in neurodegenerative disorders.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca(2+) buffering and causes neurodegeneration in primary cortical neurons.J Neurochem. 2012 Oct;123(1):147-58. doi: 10.1111/j.1471-4159.2012.07859.x. Epub 2012 Aug 14. J Neurochem. 2012. PMID: 22762283 Free PMC article.
-
Down-regulation of Homer1 attenuates t-BHP-induced oxidative stress through regulating calcium homeostasis and ER stress in brain endothelial cells.Biochem Biophys Res Commun. 2016 Sep 2;477(4):970-976. doi: 10.1016/j.bbrc.2016.07.012. Epub 2016 Jul 7. Biochem Biophys Res Commun. 2016. PMID: 27396622
-
Nuclear ALG-2 protein interacts with Ca2+ homeostasis endoplasmic reticulum protein (CHERP) Ca2+-dependently and participates in regulation of alternative splicing of inositol trisphosphate receptor type 1 (IP3R1) pre-mRNA.J Biol Chem. 2013 Nov 15;288(46):33361-75. doi: 10.1074/jbc.M113.497479. Epub 2013 Sep 27. J Biol Chem. 2013. PMID: 24078636 Free PMC article.
-
Nuclear Ca2+ signalling in cerebellar Purkinje neurons.Cerebellum. 2006;5(1):36-42. doi: 10.1080/14734220600554438. Cerebellum. 2006. PMID: 16527762 Review.
-
Inositol 1,4,5-trisphosphate receptor subtype-specific regulation of calcium oscillations.Neurochem Res. 2011 Jul;36(7):1175-85. doi: 10.1007/s11064-011-0457-7. Epub 2011 Apr 11. Neurochem Res. 2011. PMID: 21479917 Free PMC article. Review.
Cited by
-
Differential up-regulation of Vesl-1/Homer 1 protein isoforms associated with decline in visual performance in a preclinical glaucoma model.Vision Res. 2014 Jan;94:16-23. doi: 10.1016/j.visres.2013.10.018. Epub 2013 Nov 9. Vision Res. 2014. PMID: 24219919 Free PMC article.
-
The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca²⁺-release channel.Biochim Biophys Acta. 2015 Sep;1853(9):1992-2005. doi: 10.1016/j.bbamcr.2014.12.006. Epub 2014 Dec 10. Biochim Biophys Acta. 2015. PMID: 25499268 Free PMC article. Review.
-
The Spontaneous Ataxic Mouse Mutant Tippy is Characterized by a Novel Purkinje Cell Morphogenesis and Degeneration Phenotype.Cerebellum. 2015 Jun;14(3):292-307. doi: 10.1007/s12311-014-0640-x. Cerebellum. 2015. PMID: 25626522 Free PMC article.
-
Dietary Alcohol Consumption Elicits Corneal Toxicity Through the Generation of Cellular Oxidative Stress.J Ocul Pharmacol Ther. 2023 Jun;39(5):303-316. doi: 10.1089/jop.2022.0187. Epub 2023 May 29. J Ocul Pharmacol Ther. 2023. PMID: 37253141 Free PMC article.
-
Exome sequencing of case-unaffected-parents trios reveals recessive and de novo genetic variants in sporadic ALS.Sci Rep. 2015 Mar 16;5:9124. doi: 10.1038/srep09124. Sci Rep. 2015. PMID: 25773295 Free PMC article.
References
-
- Alia M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19:119–128. - PubMed
-
- Baskys A, Adamchik Y. Neuroprotective effects of extracellular glutamate are absent in hippocampal organotypic cultures treated with the amyloid peptide Abeta(25–35) Brain Res. 2001;907:188–194. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Berridge MJ. Calcium microdomains: organization and function. Cell Calcium. 2006;40:405–412. - PubMed
-
- Berridge MJ. Calcium hypothesis of Alzheimer's disease. Pflugers Arch. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG022550/AG/NIA NIH HHS/United States
- P01 AG027956/AG/NIA NIH HHS/United States
- AG022550/AG/NIA NIH HHS/United States
- P01 AG010485/AG/NIA NIH HHS/United States
- EY014227/EY/NEI NIH HHS/United States
- RR022570/RR/NCRR NIH HHS/United States
- AG010485/AG/NIA NIH HHS/United States
- R01 EY014227/EY/NEI NIH HHS/United States
- AG027956/AG/NIA NIH HHS/United States
- P20-MD001633/MD/NIMHD NIH HHS/United States
- S10 RR027093/RR/NCRR NIH HHS/United States
- S10 RR022570/RR/NCRR NIH HHS/United States
- P20 MD001633/MD/NIMHD NIH HHS/United States
- RR027093/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

