Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue?
- PMID: 21075199
- PMCID: PMC3039108
- DOI: 10.1016/j.bbi.2010.11.005
Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue?
Abstract
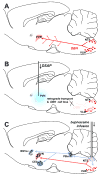
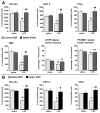
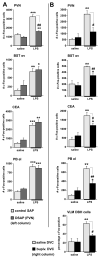
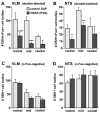
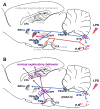
Immune challenges can lead to marked behavioral changes, including fatigue, reduced social interest, anorexia, and somnolence, but the precise neuronal mechanisms that underlie sickness behavior remain elusive. Part of the neurocircuitry influencing behavior associated with illness likely includes viscerosensory nuclei located in the caudal brainstem, based on findings that inactivation of the dorsal vagal complex (DVC) can prevent social withdrawal. These brainstem nuclei contribute multiple neuronal projections that target different components of autonomic and stress-related neurocircuitry. In particular, catecholaminergic neurons in the ventrolateral medulla (VLM) and DVC target the hypothalamus and drive neuroendocrine responses to immune challenge, but their particular role in sickness behavior is not known. To test whether this catecholamine pathway also mediates sickness behavior, we compared effects of DVC inactivation with targeted lesion of the catecholamine pathway on exploratory behavior, which provides an index of motivation and fatigue, and associated patterns of brain activation assessed by immunohistochemical detection of c-Fos protein. LPS treatment dramatically reduced exploratory behavior, and produced a pattern of increased c-Fos expression in brain regions associated with stress and autonomic adjustments paraventricular hypothalamus (PVN), bed nucleus of the stria terminalis (BST), central amygdala (CEA), whereas activation was reduced in regions involved in exploratory behavior (hippocampus, dorsal striatum, ventral tuberomammillary nucleus, and ventral tegmental area). Both DVC inactivation and catecholamine lesion prevented reductions in exploratory behavior and completely blocked the inhibitory LPS effects on c-Fos expression in the behavior-associated regions. In contrast, LPS-induced activation in the CEA and BST was inhibited by DVC inactivation but not by catecholamine lesion. The findings support the idea that parallel pathways from immune-sensory caudal brainstem sources target distinct populations of forebrain neurons that likely mediate different aspects of sickness. The caudal medullary catecholaminergic projections to the hypothalamus may significantly contribute to brain mechanisms that induce behavioral "fatigue" in the context of physiological stressors.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
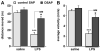





Similar articles
-
Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain.Brain Res. 2007 Jan 26;1130(1):130-45. doi: 10.1016/j.brainres.2006.10.084. Epub 2006 Dec 13. Brain Res. 2007. PMID: 17169348
-
Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-Fos expression in central autonomic nuclei.Brain Behav Immun. 2004 Mar;18(2):123-34. doi: 10.1016/j.bbi.2003.09.004. Brain Behav Immun. 2004. PMID: 14759590
-
Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling.Brain Res. 2009 Oct 19;1294:61-79. doi: 10.1016/j.brainres.2009.07.076. Epub 2009 Jul 30. Brain Res. 2009. PMID: 19646973 Free PMC article.
-
How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry.Brain Behav Immun. 2008 Nov;22(8):1160-72. doi: 10.1016/j.bbi.2008.05.001. Epub 2008 Jun 17. Brain Behav Immun. 2008. PMID: 18562160 Free PMC article.
-
Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways.Neuroscience. 2008 Mar 3;152(1):273-87. doi: 10.1016/j.neuroscience.2007.10.042. Neuroscience. 2008. PMID: 18082968 Free PMC article.
Cited by
-
Proposed toxic and hypoxic impairment of a brainstem locus in autism.Int J Environ Res Public Health. 2013 Dec 11;10(12):6955-7000. doi: 10.3390/ijerph10126955. Int J Environ Res Public Health. 2013. PMID: 24336025 Free PMC article.
-
A role for orexin in cytotoxic chemotherapy-induced fatigue.Brain Behav Immun. 2014 Mar;37:84-94. doi: 10.1016/j.bbi.2013.11.003. Epub 2013 Nov 9. Brain Behav Immun. 2014. PMID: 24216337 Free PMC article.
-
Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways.BMC Med. 2012 Jun 29;10:66. doi: 10.1186/1741-7015-10-66. BMC Med. 2012. PMID: 22747645 Free PMC article. Review.
-
Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress.Endocrinology. 2021 Nov 1;162(11):bqab185. doi: 10.1210/endocr/bqab185. Endocrinology. 2021. PMID: 34467976 Free PMC article. Review.
-
Restricted role of CRF1 receptor for the activity of brainstem catecholaminergic neurons in the negative state of morphine withdrawal.Psychopharmacology (Berl). 2012 Mar;220(2):379-93. doi: 10.1007/s00213-011-2478-y. Epub 2011 Sep 21. Psychopharmacology (Berl). 2012. PMID: 21947312
References
-
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic–pituitary–adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–11453. - PMC - PubMed
-
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

