The biological significance of methionine sulfoxide stereochemistry
- PMID: 21075204
- PMCID: PMC3311537
- DOI: 10.1016/j.freeradbiomed.2010.11.008
The biological significance of methionine sulfoxide stereochemistry
Abstract
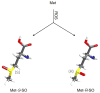
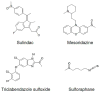
Methionine can be oxidized by reactive oxygen species to a mixture of two diastereomers, methionine-S-sulfoxide and methionine-R-sulfoxide. Both free amino acid and protein-based forms of methionine-S-sulfoxide are stereospecifically reduced by MsrA, whereas the reduction of methionine-R-sulfoxide requires two enzymes, MsrB and fRMsr, which act on its protein-based and free amino acid forms, respectively. However, mammals lack fRMsr and are characterized by deficiency in the reduction of free methionine-R-sulfoxide. The biological significance of such biased reduction of methionine sulfoxide has not been fully explored. MsrA and MsrB activities decrease during aging, leading to accumulation of protein-based and free amino acid forms of methionine sulfoxide. Since methionine is an indispensible amino acid in human nutrition and a key metabolite in sulfur, methylation, and transsulfuration pathways, the consequences of accumulation of its oxidized forms require further studies. Finally, in addition to methionine, methylsulfinyl groups are present in various drugs and natural compounds, and their differential reduction by Msrs may have important therapeutic implications.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Functional analysis of free methionine-R-sulfoxide reductase from Saccharomyces cerevisiae.J Biol Chem. 2009 Feb 13;284(7):4354-64. doi: 10.1074/jbc.M805891200. Epub 2008 Dec 2. J Biol Chem. 2009. PMID: 19049972 Free PMC article.
-
Methionine sulfoxide reduction in mammals: characterization of methionine-R-sulfoxide reductases.Mol Biol Cell. 2004 Mar;15(3):1055-64. doi: 10.1091/mbc.e03-08-0629. Epub 2003 Dec 29. Mol Biol Cell. 2004. PMID: 14699060 Free PMC article.
-
Repair of oxidized proteins. Identification of a new methionine sulfoxide reductase.J Biol Chem. 2001 Dec 28;276(52):48915-20. doi: 10.1074/jbc.M105509200. Epub 2001 Oct 24. J Biol Chem. 2001. PMID: 11677230
-
Functions and evolution of selenoprotein methionine sulfoxide reductases.Biochim Biophys Acta. 2009 Nov;1790(11):1471-7. doi: 10.1016/j.bbagen.2009.04.014. Epub 2009 May 4. Biochim Biophys Acta. 2009. PMID: 19406207 Free PMC article. Review.
-
Roles of methionine suldfoxide reductases in antioxidant defense, protein regulation and survival.Curr Pharm Des. 2005;11(11):1451-7. doi: 10.2174/1381612053507846. Curr Pharm Des. 2005. PMID: 15853675 Review.
Cited by
-
Feeding broiler chickens with arginine above recommended levels: effects on growth performance, metabolism, and intestinal microbiota.J Anim Sci Biotechnol. 2023 Mar 3;14(1):33. doi: 10.1186/s40104-023-00839-y. J Anim Sci Biotechnol. 2023. PMID: 36864475 Free PMC article.
-
Methionine Oxidation Changes the Mechanism of Aβ Peptide Binding to the DMPC Bilayer.Sci Rep. 2019 Apr 11;9(1):5947. doi: 10.1038/s41598-019-42304-9. Sci Rep. 2019. PMID: 30976055 Free PMC article.
-
Methionine sulfoxide reductase B from Corynebacterium diphtheriae catalyzes sulfoxide reduction via an intramolecular disulfide cascade.J Biol Chem. 2020 Mar 13;295(11):3664-3677. doi: 10.1074/jbc.RA119.012438. Epub 2020 Jan 28. J Biol Chem. 2020. PMID: 31992594 Free PMC article.
-
Oxidative stress, protein damage and repair in bacteria.Nat Rev Microbiol. 2017 Jul;15(7):385-396. doi: 10.1038/nrmicro.2017.26. Epub 2017 Apr 19. Nat Rev Microbiol. 2017. PMID: 28420885 Review.
-
Effects of E-Cigarettes on the Lung and Systemic Metabolome in People with HIV.Metabolites. 2024 Aug 6;14(8):434. doi: 10.3390/metabo14080434. Metabolites. 2024. PMID: 39195530 Free PMC article.
References
-
- Metayer S, Seiliez I, Collin A, Duchene S, Mercier Y, Geraert PA, Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem. 2008;19:207–215. - PubMed
-
- Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29:190–201. - PubMed
-
- Zou CG, Banerjee R. Homocysteine and redox signaling. Antioxid Redox Signal. 2005;7:547–559. - PubMed
-
- Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

