Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart
- PMID: 21075216
- PMCID: PMC3138056
- DOI: 10.1016/j.hrthm.2010.11.019
Morphologic pattern of the intrinsic ganglionated nerve plexus in mouse heart
Abstract
Background: Both normal and genetically modified mice are excellent models for investigating molecular mechanisms of arrhythmogenic cardiac diseases that may be associated with an imbalance between sympathetic and parasympathetic nervous input to the heart.
Objective: The purpose of this study was to (1) determine the structural organization of the mouse cardiac neural plexus, (2) identify extrinsic neural sources and their relationship with the cardiac plexus, and (3) reveal any anatomic differences in the cardiac plexus between mouse and other species.
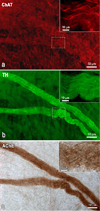
Methods: Cardiac nerve structures were visualized using histochemical staining for acetylcholinesterase (AChE) on whole heart and thorax-dissected preparations derived from 25 mice. To confirm the reliability of staining parasympathetic and sympathetic neural components in the mouse heart, we applied a histochemical method for AChE and immunohistochemistry for tyrosine hydroxylase (TH) and/or choline acetyltransferase (ChAT) on whole mounts preparations from six mice.
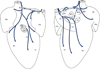
Results: Double immunohistochemical labeling of TH and ChAT on AChE-positive neural elements in mouse whole mounts demonstrated equal staining of nerves and ganglia for AChE that were positive for both TH and ChAT. The extrinsic cardiac nerves access the mouse heart at the right and left cranial veins and interblend within the ganglionated nerve plexus of the heart hilum that is persistently localized on the heart base. Nerves and bundles of nerve fibers extend epicardially from this plexus to atria and ventricles by left dorsal, dorsal right atrial, right ventral, and ventral left atrial routes or subplexuses. The right cranial vein receives extrinsic nerves that mainly originate from the right cervicothoracic ganglion and a branch of the right vagus nerve, whereas the left cranial vein is supplied by extrinsic nerves from the left cervicothoracic ganglion and the left vagus nerve. The majority of intrinsic cardiac ganglia are localized on the heart base at the roots of the pulmonary veins. These ganglia are interlinked by interganglionic nerves into the above mentioned nerve plexus of the heart hilum. In general, the examined hearts contained 19 ± 3 ganglia, giving a cumulative ganglion area of 0.4 ± 0.1 mm(2).
Conclusion: Despite substantial anatomic differences in ganglion number and distribution, the structural organization of the intrinsic ganglionated plexus in the mouse heart corresponds in general to that of other mammalian species, including human.
Copyright © 2011 Heart Rhythm Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No Conflicts
Figures



Similar articles
-
Epicardial neural ganglionated plexus of ovine heart: anatomic basis for experimental cardiac electrophysiology and nerve protective cardiac surgery.Heart Rhythm. 2010 Jul;7(7):942-50. doi: 10.1016/j.hrthm.2010.02.036. Epub 2010 Mar 1. Heart Rhythm. 2010. PMID: 20197118 Free PMC article.
-
Morphological pattern of intrinsic nerve plexus distributed on the rabbit heart and interatrial septum.J Anat. 2014 May;224(5):583-93. doi: 10.1111/joa.12166. Epub 2014 Feb 14. J Anat. 2014. PMID: 24527844 Free PMC article.
-
Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations.Heart Rhythm. 2011 May;8(5):731-8. doi: 10.1016/j.hrthm.2011.01.013. Epub 2011 Jan 11. Heart Rhythm. 2011. PMID: 21232628 Free PMC article.
-
The heart's 'little brain' controlling cardiac function in the rabbit.Exp Physiol. 2015 Apr 1;100(4):348-53. doi: 10.1113/expphysiol.2014.080168. Epub 2014 Oct 29. Exp Physiol. 2015. PMID: 25833107 Free PMC article. Review.
-
Intrinsic cardiac autonomic nervous system: What do clinical electrophysiologists need to know about the "heart brain"?J Cardiovasc Electrophysiol. 2021 Jun;32(6):1737-1747. doi: 10.1111/jce.15058. Epub 2021 May 5. J Cardiovasc Electrophysiol. 2021. PMID: 33928710 Review.
Cited by
-
IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis.Clin Exp Immunol. 2020 Mar;199(3):303-313. doi: 10.1111/cei.13401. Epub 2019 Dec 9. Clin Exp Immunol. 2020. PMID: 31758701 Free PMC article.
-
The nervous heart.Prog Biophys Mol Biol. 2016 Jan;120(1-3):199-209. doi: 10.1016/j.pbiomolbio.2015.12.015. Epub 2016 Jan 11. Prog Biophys Mol Biol. 2016. PMID: 26780507 Free PMC article. Review.
-
Autonomic Modulation for Cardiovascular Disease.Front Physiol. 2020 Dec 22;11:617459. doi: 10.3389/fphys.2020.617459. eCollection 2020. Front Physiol. 2020. PMID: 33414727 Free PMC article. Review.
-
SHP-2 deletion in postmigratory neural crest cells results in impaired cardiac sympathetic innervation.Proc Natl Acad Sci U S A. 2014 Apr 8;111(14):E1374-82. doi: 10.1073/pnas.1319208111. Epub 2014 Mar 24. Proc Natl Acad Sci U S A. 2014. PMID: 24706815 Free PMC article.
-
Topographical mapping of catecholaminergic axon innervation in the flat-mounts of the mouse atria: a quantitative analysis.Sci Rep. 2023 Apr 7;13(1):4850. doi: 10.1038/s41598-023-27727-9. Sci Rep. 2023. PMID: 37029119 Free PMC article.
References
-
- Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol. 2000;89:1903–1911. - PubMed
-
- Tsuboi M, Furukawa Y, Nakajima K, Kurogouchi F, Chiba S. Inotropic, chronotropic, and dromotropic effects mediated via parasympathetic ganglia in the dog heart. Am J Physiol Heart Circ Physiol. 2000;279:H1201–H1207. - PubMed
-
- Cifelli C, Rose RA, Zhang H, et al. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–535. - PubMed
-
- Gatti PJ, Johnson TA, Phan P, Jordan IK, 3rd, Coleman W, Massari VJ. The physiological and anatomical demonstration of functionally selective parasympathetic ganglia located in discrete fat pads on the feline myocardium. J Auton Nerv Syst. 1995;51:255–259. - PubMed
-
- Armour JA. Potential clinical relevance of the 'little brain' on the mammalian heart. Exp Physiol. 2008;93:165–176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

