Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear
- PMID: 21075228
- PMCID: PMC2987592
- DOI: 10.1016/j.biopsych.2010.09.004
Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear
Abstract
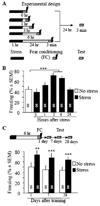
Background: Glutamatergic transmission is one of the main components of the stress response; nevertheless, its role in the emotional stress sequelae is not known. Here, we investigated whether interactions between group I metabotropic glutamate receptors (metabotropic glutamate receptor 1 and metabotropic glutamate receptor 5 [mGluR5]) and Homer proteins mediate the delayed and persistent enhancement of fear induced by acute stress.
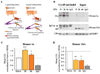
Methods: Antagonists and inverse agonists of metabotropic glutamate receptor 1 and mGluR5 were injected into the hippocampus after immobilization stress and before contextual fear conditioning. Metabotropic glutamate receptor 5 was displaced from constitutive Homer scaffolds by viral transfection of Homer1a or injection of Tat decoy peptides. The effects of these manipulations on stress-enhanced fear were determined.
Results: We show that stress induces interactions between hippocampal mGluR5 and Homer1a; causes a sustained, ligand-independent mGluR5 activity; and enhances contextual fear. Consistent with this mechanism, enhancement of fear was abolished by delayed poststress application of inverse agonists, but not antagonists, of mGluR5. The effect of stress was mimicked by virally transfected Homer1a or injection of Tat-metabotropic glutamate receptor C-tail decoy peptides into the hippocampus.
Conclusions: Constitutive activation of mGluR5 is identified as a principal hippocampal mechanism underlying the delayed stress effects on emotion and memory. Inverse agonists, but not antagonists, of mGluR5 are therefore proposed as a preventive treatment option for acute and posttraumatic stress disorders.
Copyright © 2010 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures


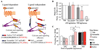

Comment in
-
An odyssey of fear: Homer stresses new mechanisms.Biol Psychiatry. 2010 Dec 1;68(11):980-1. doi: 10.1016/j.biopsych.2010.10.005. Biol Psychiatry. 2010. PMID: 21075227 Free PMC article. No abstract available.
Similar articles
-
Homer1 mediates acute stress-induced cognitive deficits in the dorsal hippocampus.J Neurosci. 2013 Feb 27;33(9):3857-64. doi: 10.1523/JNEUROSCI.4333-12.2013. J Neurosci. 2013. PMID: 23447597 Free PMC article.
-
Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation.J Neurosci. 2000 Dec 1;20(23):8710-6. doi: 10.1523/JNEUROSCI.20-23-08710.2000. J Neurosci. 2000. PMID: 11102477 Free PMC article.
-
Homer1/mGluR5 activity moderates vulnerability to chronic social stress.Neuropsychopharmacology. 2015 Mar 13;40(5):1222-33. doi: 10.1038/npp.2014.308. Neuropsychopharmacology. 2015. PMID: 25409593 Free PMC article.
-
The role of metabotropic glutamate receptor 5 in learning and memory processes.Drug News Perspect. 2005 Jul-Aug;18(6):353-61. doi: 10.1358/dnp.2005.18.6.927927. Drug News Perspect. 2005. PMID: 16247513 Review.
-
Metabotropic glutamate receptor subtype 5 antagonism in learning and memory.Eur J Pharmacol. 2010 Aug 10;639(1-3):17-25. doi: 10.1016/j.ejphar.2009.12.039. Epub 2010 Apr 2. Eur J Pharmacol. 2010. PMID: 20363219 Free PMC article. Review.
Cited by
-
Metabotropic Glutamatergic Receptor 5 and Stress Disorders: Knowledge Gained From Receptor Imaging Studies.Biol Psychiatry. 2018 Jul 15;84(2):95-105. doi: 10.1016/j.biopsych.2017.08.025. Epub 2017 Sep 19. Biol Psychiatry. 2018. PMID: 29100629 Free PMC article. Review.
-
Preso1 dynamically regulates group I metabotropic glutamate receptors.Nat Neurosci. 2012 Jun;15(6):836-44. doi: 10.1038/nn.3103. Nat Neurosci. 2012. PMID: 22561452 Free PMC article.
-
Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain.Front Pharmacol. 2012 Oct 12;3:182. doi: 10.3389/fphar.2012.00182. eCollection 2012. Front Pharmacol. 2012. PMID: 23091460 Free PMC article.
-
Modulation of behavior by scaffolding proteins of the post-synaptic density.Neurobiol Learn Mem. 2013 Oct;105:3-12. doi: 10.1016/j.nlm.2013.04.014. Epub 2013 May 20. Neurobiol Learn Mem. 2013. PMID: 23701866 Free PMC article. Review.
-
Pharmacotherapy for Co-Occurring Alcohol Use Disorder and Post-Traumatic Stress Disorder: Targeting the Opioidergic, Noradrenergic, Serotonergic, and GABAergic/Glutamatergic Systems.Alcohol Res. 2018;39(2):193-205. Alcohol Res. 2018. PMID: 31198658 Free PMC article. Review.
References
-
- Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, et al. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393–405. - PubMed
-
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. - PubMed
-
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. - PubMed
-
- Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003;23:700–707. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

