Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells
- PMID: 21075310
- PMCID: PMC3397918
- DOI: 10.1016/j.ccr.2010.10.020
Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells
Abstract
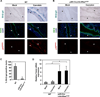
Mutational activation of KRas is the first and most frequently detected genetic lesion in pancreatic ductal adenocarcinoma (PDAC). However, the precise role of oncogenic KRas in the pathogenesis of PDAC is not fully understood. Here, we report that the endogenous expression of oncogenic KRas suppresses premature senescence in primary pancreatic duct epithelial cells (PDEC). Oncogenic KRas-mediated senescence bypass is conferred by the upregulation of the basic helix-loop-helix transcription factor Twist that in turn abrogates p16(INK4A) induction. Moreover, the KRas-Twist-p16(INK4A) senescence bypass pathway is employed in vivo to prevent inflammation-associated senescence of pancreatic ductal epithelium. Our findings indicate that oncogenic KRas could contribute to PDAC initiation by protecting cells from entering a state of permanent growth arrest.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





References
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
-
- Agbunag C, Bar-Sagi D. Oncogenic K-ras drives cell cycle progression and phenotypic conversion of primary pancreatic duct epithelial cells. Cancer Res. 2004;64:5659–5663. - PubMed
-
- Agbunag C, Lee KE, Buontempo S, Bar-Sagi D. Pancreatic duct epithelial cell isolation and cultivation in two-dimensional and three-dimensional culture systems. Methods Enzymol. 2006;407:703–710. - PubMed
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–554. - PubMed
-
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

