Statins enhance formation of phagocyte extracellular traps
- PMID: 21075355
- PMCID: PMC3008410
- DOI: 10.1016/j.chom.2010.10.005
Statins enhance formation of phagocyte extracellular traps
Abstract
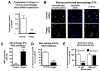
Statins are inhibitors of 3-hydroxy 3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis. Recent clinico-epidemiologic studies correlate patients receiving statin therapy with having reduced mortality associated with severe bacterial infection. Investigating the effect of statins on the innate immune capacity of phagocytic cells against the human pathogen Staphylococcus aureus, we uncovered a beneficial effect of statins on bacterial clearance by phagocytes, although, paradoxically, both phagocytosis and oxidative burst were inhibited. Probing instead for an extracellular mechanism of killing, we found that statins boosted the production of antibacterial DNA-based extracellular traps (ETs) by human and murine neutrophils and also monocytes/macrophages. The effect of statins to induce phagocyte ETs was linked to sterol pathway inhibition. We conclude that a drug therapy taken chronically by millions alters the functional behavior of phagocytic cells, which could have ramifications for susceptibility and response to bacterial infections in these patients.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures







References
-
- Almog Y, Novack V, Eisinger M, Porath A, Novack L, Gilutz H. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med. 2007;35:372–378. - PubMed
-
- Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046. - PubMed
-
- Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. - PubMed
-
- Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. - PubMed
-
- Benati D, Ferro M, Savino MT, Ulivieri C, Schiavo E, Nuccitelli A, Pasini FL, Baldari CT. Opposite effects of simvastatin on the bactericidal and inflammatory response of macrophages to opsonized S. aureus. J Leukoc Biol 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

