Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice
- PMID: 21075356
- PMCID: PMC3032408
- DOI: 10.1016/j.chom.2010.10.004
Anthrax toxin targeting of myeloid cells through the CMG2 receptor is essential for establishment of Bacillus anthracis infections in mice
Abstract
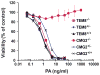
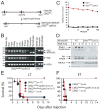
Bacillus anthracis kills through a combination of bacterial infection and toxemia. Anthrax toxin working via the CMG2 receptor mediates lethality late in infection, but its roles early in infection remain unclear. We generated myeloid-lineage specific CMG2-deficient mice to examine the roles of macrophages, neutrophils, and other myeloid cells in anthrax pathogenesis. Macrophages and neutrophils isolated from these mice were resistant to anthrax toxin. However, the myeloid-specific CMG2-deficient mice remained fully sensitive to both anthrax lethal and edema toxins, demonstrating that targeting of myeloid cells is not responsible for anthrax toxin-induced lethality. Surprisingly, the myeloid-specific CMG2-deficient mice were completely resistant to B. anthracis infection. Neutrophil depletion experiments suggest that B. anthracis relies on anthrax toxin secretion to evade the scavenging functions of neutrophils to successfully establish infection. This work demonstrates that anthrax toxin uptake through CMG2 and the resulting impairment of myeloid cells are essential to anthrax infection.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Anthrax toxin delivers a one-two punch.Cell Host Microbe. 2010 Nov 18;8(5):394-5. doi: 10.1016/j.chom.2010.10.011. Cell Host Microbe. 2010. PMID: 21075350 Free PMC article.
References
-
- Arora N, Leppla SH. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. - PubMed
-
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. - PubMed
-
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. - PubMed
-
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

