ERAD and ERAD tuning: disposal of cargo and of ERAD regulators from the mammalian ER
- PMID: 21075612
- PMCID: PMC7172097
- DOI: 10.1016/j.ceb.2010.10.002
ERAD and ERAD tuning: disposal of cargo and of ERAD regulators from the mammalian ER
Abstract
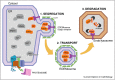
The endoplasmic reticulum (ER) is the site of maturation for secretory and membrane proteins in eukaryotic cells. Unsuccessful folding attempts are eventually interrupted and most folding-defective polypeptides are dislocated across the ER membrane and degraded by cytosolic proteasomes in a complex series of events collectively defined as ER-associated degradation (ERAD). Uncontrolled ERAD activity might prematurely interrupt ongoing folding programs. At steady state, this is prevented by ERAD tuning, that is, the removal of select ERAD regulators from the ER and their degradation by proteasomes and by endo-lysosomal proteases. In Coronaviruses infected cells, the formation of LC3-I coated vesicles containing ERAD regulators cleared from the ER lumen is co-opted to anchor viral replication and transcription complexes to ER-derived membranes.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Role of the SEL1L:LC3-I complex as an ERAD tuning receptor in the mammalian ER.Mol Cell. 2012 Jun 29;46(6):809-19. doi: 10.1016/j.molcel.2012.04.017. Epub 2012 May 24. Mol Cell. 2012. Retraction in: Mol Cell. 2014 Dec 18;56(6):819. doi: 10.1016/j.molcel.2014.11.029. PMID: 22633958 Retracted.
-
Unconventional roles of nonlipidated LC3 in ERAD tuning and coronavirus infection.Autophagy. 2012 Oct;8(10):1534-6. doi: 10.4161/auto.21229. Epub 2012 Aug 16. Autophagy. 2012. PMID: 22895348
-
Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways.Crit Rev Biochem Mol Biol. 2019 Apr;54(2):153-163. doi: 10.1080/10409238.2019.1610351. Epub 2019 May 14. Crit Rev Biochem Mol Biol. 2019. PMID: 31084437 Review.
-
The reductase TMX1 contributes to ERAD by preferentially acting on membrane-associated folding-defective polypeptides.Biochem Biophys Res Commun. 2018 Sep 5;503(2):938-943. doi: 10.1016/j.bbrc.2018.06.099. Epub 2018 Jun 22. Biochem Biophys Res Commun. 2018. PMID: 29932915
-
ER-to-lysosome-associated degradation in a nutshell: mammalian, yeast, and plant ER-phagy as induced by misfolded proteins.FEBS Lett. 2023 Aug;597(15):1928-1945. doi: 10.1002/1873-3468.14674. Epub 2023 Jun 10. FEBS Lett. 2023. PMID: 37259628 Review.
Cited by
-
The lifetime of UDP-galactose:ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones.J Biol Chem. 2012 Dec 14;287(51):43156-69. doi: 10.1074/jbc.M112.380444. Epub 2012 Oct 26. J Biol Chem. 2012. PMID: 23105111 Free PMC article.
-
Arms Race between Enveloped Viruses and the Host ERAD Machinery.Viruses. 2016 Sep 19;8(9):255. doi: 10.3390/v8090255. Viruses. 2016. PMID: 27657106 Free PMC article. Review.
-
SARS-CoV-2 ORF8 and SARS-CoV ORF8ab: Genomic Divergence and Functional Convergence.Pathogens. 2020 Aug 20;9(9):677. doi: 10.3390/pathogens9090677. Pathogens. 2020. PMID: 32825438 Free PMC article. Review.
-
The Best for the Most Important: Maintaining a Pristine Proteome in Stem and Progenitor Cells.Stem Cells Int. 2019 May 2;2019:1608787. doi: 10.1155/2019/1608787. eCollection 2019. Stem Cells Int. 2019. PMID: 31191665 Free PMC article. Review.
-
Golgi Alpha1,2-Mannosidase IA Promotes Efficient Endoplasmic Reticulum-Associated Degradation of NKCC2.Cells. 2021 Dec 29;11(1):101. doi: 10.3390/cells11010101. Cells. 2021. PMID: 35011665 Free PMC article.
References
-
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. - PubMed
-
- Aebi M., Bernasconi R., Clerc S., Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 2010;35:74–82. - PubMed
-
- Wilson C.M., High S. Ribophorin I acts as a substrate-specific facilitator of N-glycosylation. J Cell Sci. 2007;120:648–657. - PubMed
-
- Schulz B.L., Stirnimann C.U., Grimshaw J.P., Brozzo M.S., Fritsch F., Mohorko E., Capitani G., Glockshuber R., Grutter M.G., Aebi M. Oxidoreductase activity of oligosaccharyltransferase subunits Ost3p and Ost6p defines site-specific glycosylation efficiency. Proc Natl Acad Sci U S A. 2009;106:11061–11066. - PMC - PubMed
-
- Zapun A., Darby N.J., Tessier D.C., Michalak M., Bergeron J.J., Thomas D.Y. Enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem. 1998;273:6009–6012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

