Inhibition of proprotein convertase SKI-1 blocks transcription of key extracellular matrix genes regulating osteoblastic mineralization
- PMID: 21075843
- PMCID: PMC3023479
- DOI: 10.1074/jbc.M110.151647
Inhibition of proprotein convertase SKI-1 blocks transcription of key extracellular matrix genes regulating osteoblastic mineralization
Abstract
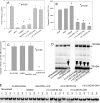
Mineralization, a characteristic phenotypic property of osteoblastic lineage cells, was blocked by 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) and decanoyl-Arg-Arg-Leu-Leu-chloromethyl ketone (dec-RRLL-cmk), inhibitors of SKI-1 (site 1; subtilisin kexin like-1) protease. Because SKI-1 is required for activation of SREBP and CREB (cAMP-response element-binding protein)/ATF family transcription factors, we tested the effect of these inhibitors on gene expression. AEBSF decreased expression of 140 genes by 1.5-3.0-fold including Phex, Dmp1, COL1A1, COL11A1, and fibronectin. Direct comparison of AEBSF and dec-RRLL-cmk, a more specific SKI-1 inhibitor, demonstrated that expression of Phex, Dmp1, COL11A1, and fibronectin was reduced by both, whereas COL1A2 and HMGCS1 were reduced only by AEBSF. AEBSF and dec-RRLL-cmk decreased the nuclear content of SKI-1-activated forms of transcription factors SREBP-1, SREBP-2, and OASIS. In contrast to AEBSF, the actions of dec-RRLL-cmk represent the sum of its direct actions on SKI-1 and indirect actions on caspase-3. Specifically, dec-RRLL-cmk reduced intracellular caspase-3 activity by blocking the formation of activated 19-kDa caspase-3. Conversely, overexpression of SKI-1-activated SREBP-1a and CREB-H in UMR106-01 osteoblastic cells increased the number of mineralized foci and altered their morphology to yield mineralization nodules, respectively. In summary, SKI-1 regulates the activation of transmembrane transcription factor precursors required for expression of key genes required for mineralization of osteoblastic cultures in vitro and bone formation in vivo. Our results indicate that the differentiated phenotype of osteoblastic cells and possibly osteocytes depends upon the non-apoptotic actions of SKI-1.
Figures







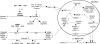
Similar articles
-
Inhibition of the prohormone convertase subtilisin-kexin isoenzyme-1 induces apoptosis in human melanoma cells.J Invest Dermatol. 2014 Jan;134(1):168-175. doi: 10.1038/jid.2013.282. Epub 2013 Jun 27. J Invest Dermatol. 2014. PMID: 23884247
-
Potential role of proprotein convertase SKI-1 in the mineralization of primary bone.Cells Tissues Organs. 2009;189(1-4):25-32. doi: 10.1159/000151723. Epub 2008 Aug 26. Cells Tissues Organs. 2009. PMID: 18728345 Free PMC article.
-
Association of specific proteolytic processing of bone sialoprotein and bone acidic glycoprotein-75 with mineralization within biomineralization foci.J Biol Chem. 2007 Sep 7;282(36):26002-13. doi: 10.1074/jbc.M701332200. Epub 2007 Jul 5. J Biol Chem. 2007. PMID: 17613519 Free PMC article.
-
Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway.Crit Rev Eukaryot Gene Expr. 2012;22(1):61-86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. Crit Rev Eukaryot Gene Expr. 2012. PMID: 22339660 Free PMC article. Review.
-
[Osteocyte and DMP1].Clin Calcium. 2012 May;22(5):713-20. Clin Calcium. 2012. PMID: 22549196 Review. Japanese.
Cited by
-
Type IV collagen is a novel DEJ biomarker that is reduced by radiotherapy.J Dent Res. 2014 Oct;93(10):1028-34. doi: 10.1177/0022034514548221. Epub 2014 Aug 21. J Dent Res. 2014. PMID: 25146181 Free PMC article.
-
Transcription factor old astrocyte specifically induced substance is a novel regulator of kidney fibrosis.FASEB J. 2021 Feb;35(2):e21158. doi: 10.1096/fj.202001820R. Epub 2020 Nov 5. FASEB J. 2021. PMID: 33150680 Free PMC article.
-
Biomineralization of bone: a fresh view of the roles of non-collagenous proteins.Front Biosci (Landmark Ed). 2011 Jun 1;16(7):2598-621. doi: 10.2741/3875. Front Biosci (Landmark Ed). 2011. PMID: 21622198 Free PMC article. Review.
-
Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) Gene in Osteocytes Stimulates Soleus Muscle Regeneration and Increased Size and Contractile Force with Age.J Biol Chem. 2016 Feb 26;291(9):4308-22. doi: 10.1074/jbc.M115.686626. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719336 Free PMC article.
-
Osteocyte regulation of bone mineral: a little give and take.Osteoporos Int. 2012 Aug;23(8):2067-79. doi: 10.1007/s00198-012-1915-z. Osteoporos Int. 2012. PMID: 22302104 Review.
References
-
- Lian J. B., Stein G. S. (1992) Crit. Rev. Oral Biol. Med. 3, 269–305 - PubMed
-
- Ruchon A. F., Tenenhouse H. S., Marcinkiewicz M., Siegfried G., Aubin J. E., DesGroseillers L., Crine P., Boileau G. (2000) J. Bone Miner. Res. 15, 1440–1450 - PubMed
-
- The HYP Consortium (1995) Nat. Genet. 11, 130–136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

