The proprotein convertase PC7: unique zymogen activation and trafficking pathways
- PMID: 21075846
- PMCID: PMC3024769
- DOI: 10.1074/jbc.M110.192344
The proprotein convertase PC7: unique zymogen activation and trafficking pathways
Abstract
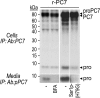
The zymogen activation mechanism and physiological functions of the most ancient and highly conserved basic amino acid-specific proprotein convertase 7 (PC7) are not known. Herein, we characterized the biosynthesis, subcellular localization, and trafficking of the membrane-bound full-length rat and human PC7. The prosegment of PC7 is primarily secreted alone as a non-inhibitory protein via the conventional, Golgi-dependent, secretory pathway. Mature PC7 is partially sulfated and thus reaches the cell surface via the conventional route. However, a fraction of PC7 reaches the cell surface through a brefeldin A- and COPII-independent unconventional secretory pathway. The latter trafficking may explain the rapid (<10 min) transit of a fraction of PC7 from the ER to the cell surface. Electron microscopy further confirmed the localization of PC7 to the cell surface of HEK293 cells. Within the cytosolic tail, only two cysteines (Cys(699) and Cys(704)) are palmitoylated, but this modification does not affect the choice of trafficking pathway. Swapping the transmembrane-cytosolic tail (TMCT) sequences of the convertases Furin and PC7 revealed that PC7(TMCT-Furin) is much more sulfated and hence traffics more efficiently through the conventional secretory pathway. In contrast, the Furin(TMCT-PC7) is no longer sulfated and thus reaches the cell surface by the unconventional pathway. Because trafficking of PC7(CT-Furin) and Furin(CT-PC7) resemble their wild type counterparts, we deduce that the transmembrane domain of PC7 regulates the sorting of PC7 toward the unconventional secretory pathway. In conclusion, PC7 is distinct from other proprotein convertases in its zymogen activation, subcellular localization, and trafficking.
Figures







References
-
- Seidah N. G., Prat A. (2007) J. Mol. Med. 85, 685–696 - PubMed
-
- Seidah N. G., Mayer G., Zaid A., Rousselet E., Nassoury N., Poirier S., Essalmani R., Prat A. (2008) Int. J. Biochem. Cell Biol. 40, 1111–1125 - PubMed
-
- Seidah N. G., Chrétien M. (1999) Brain Res. 848, 45–62 - PubMed
-
- Creemers J. W., Khatib A. M. (2008) Front Biosci. 13, 4960–4971 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

