Roles of Dim2 in ribosome assembly
- PMID: 21075849
- PMCID: PMC3024753
- DOI: 10.1074/jbc.M110.191494
Roles of Dim2 in ribosome assembly
Abstract
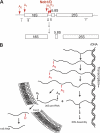
In eukaryotes, ribosome assembly requires hundreds of conserved essential proteins not present in the mature particle. Despite their importance, the function of most factors remains unknown. This is because protein deletion often affects the composition of the entire particle. Additionally, many proteins are present in assembling ribosomes for extended times, which makes it difficult to pinpoint their role to a particular step. Here we have combined classical yeast biochemistry with experiments using recombinant proteins and RNA to study the role of Dim2 and its interaction with Nob1, the nuclease that generates the 3'-end of 18 S rRNA. Analysis of Dim2 mutants in which the interaction with Nob1 is disrupted demonstrates that this interaction between Dim2 and Nob1 is essential for optimal growth, and RNA binding experiments show that Dim2 increases Nob1 RNA affinity. Furthermore, our data indicate that Dim2 helps regulate Nob1 cleavage activity at the 3'-end of 18 S rRNA, as point mutants where this interaction is abolished in vitro accumulate pre-ribosomes containing Nob1 and 20 S rRNA in vivo. Interestingly, the site of interaction with Nob1 is mapped to the canonical RNA binding surface of a KH-like domain in Dim2, providing another example where an RNA-binding domain can be repurposed for protein interactions.
Figures

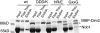




Similar articles
-
Nob1 binds the single-stranded cleavage site D at the 3'-end of 18S rRNA with its PIN domain.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14259-64. doi: 10.1073/pnas.0905403106. Epub 2009 Aug 14. Proc Natl Acad Sci U S A. 2009. PMID: 19706509 Free PMC article.
-
The kinase Rio1 and a ribosome collision-dependent decay pathway survey the integrity of 18S rRNA cleavage.PLoS Biol. 2024 Apr 25;22(4):e3001767. doi: 10.1371/journal.pbio.3001767. eCollection 2024 Apr. PLoS Biol. 2024. PMID: 39038273 Free PMC article.
-
Interaction between ribosome assembly factors Krr1 and Faf1 is essential for formation of small ribosomal subunit in yeast.J Biol Chem. 2014 Aug 15;289(33):22692-22703. doi: 10.1074/jbc.M114.584490. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990943 Free PMC article.
-
Saccharomyces cerevisiae, a Powerful Model for Studying rRNA Modifications and Their Effects on Translation Fidelity.Int J Mol Sci. 2021 Jul 10;22(14):7419. doi: 10.3390/ijms22147419. Int J Mol Sci. 2021. PMID: 34299038 Free PMC article. Review.
-
Ribosome Biogenesis and Cancer: Insights into NOB1 and PNO1 Mechanisms.Curr Pharm Des. 2024;30(37):2911-2921. doi: 10.2174/0113816128301870240730071910. Curr Pharm Des. 2024. PMID: 39143880 Review.
Cited by
-
Identification of distinct maturation steps involved in human 40S ribosomal subunit biosynthesis.Nat Commun. 2020 Jan 9;11(1):156. doi: 10.1038/s41467-019-13990-w. Nat Commun. 2020. PMID: 31919354 Free PMC article.
-
Ribosome assembly factors prevent premature translation initiation by 40S assembly intermediates.Science. 2011 Sep 9;333(6048):1449-53. doi: 10.1126/science.1208245. Epub 2011 Aug 11. Science. 2011. PMID: 21835981 Free PMC article.
-
Pre-ribosomal particles from nucleoli to cytoplasm.Nucleus. 2024 Dec;15(1):2373052. doi: 10.1080/19491034.2024.2373052. Epub 2024 Jun 28. Nucleus. 2024. PMID: 38940456 Free PMC article. Review.
-
The DEAD-box Protein Rok1 Orchestrates 40S and 60S Ribosome Assembly by Promoting the Release of Rrp5 from Pre-40S Ribosomes to Allow for 60S Maturation.PLoS Biol. 2016 Jun 9;14(6):e1002480. doi: 10.1371/journal.pbio.1002480. eCollection 2016 Jun. PLoS Biol. 2016. PMID: 27280440 Free PMC article.
-
Quality control mechanisms during ribosome maturation.Trends Cell Biol. 2013 May;23(5):242-50. doi: 10.1016/j.tcb.2013.01.004. Epub 2013 Feb 1. Trends Cell Biol. 2013. PMID: 23375955 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

