Effects of ionic strength on bacteriophage MS2 behavior and their implications for the assessment of virus retention by ultrafiltration membranes
- PMID: 21075898
- PMCID: PMC3019717
- DOI: 10.1128/AEM.01075-10
Effects of ionic strength on bacteriophage MS2 behavior and their implications for the assessment of virus retention by ultrafiltration membranes
Abstract
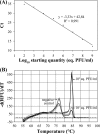
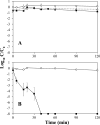
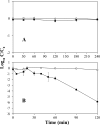
Bacteriophage MS2 is widely used as a surrogate to estimate pathogenic virus elimination by membrane filtration processes used in water treatment. Given that this water technology may be conducted with different types of waters, we focused on investigating the effects of ionic strength on MS2 behavior. For this, MS2 was analyzed while suspended in solutions of various ionic strengths, first in a batch experiment and second during membrane ultrafiltration, and quantified using (i) quantitative reverse transcriptase PCR (qRT-PCR), which detects the total number of viral genomes, (ii) qRT-PCR without the RNA extraction step, which reflects only particles with a broken capsid (free RNA), and (iii) the PFU method, which detects only infectious viruses. At the beginning of the batch experiments using solutions containing small amounts of salts, losses of MS2 infectivity (90%) and broken particles (20%) were observed; these proportions did not change during filtration. In contrast, in high-ionic-strength solutions, bacteriophage kept its biological activity under static conditions, but it quickly lost its infectivity during the filtration process. Increasing the ionic strength decreased both the inactivation and the capsid breakup in the feed suspension and increased the loss of infectivity in the filtration retentate, while the numbers of MS2 genomes were identical in both experiments. In conclusion, the effects of ionic strength on MS2 behavior may significantly distort the results of membrane filtration processes, and therefore, the combination of classical and molecular methods used here is useful for an effective validation of the retention efficiency of ultrafiltration membranes.
Figures



Similar articles
-
Removal of MS2, Qβ and GA bacteriophages during drinking water treatment at pilot scale.Water Res. 2012 May 15;46(8):2651-64. doi: 10.1016/j.watres.2012.02.020. Epub 2012 Mar 3. Water Res. 2012. PMID: 22421032
-
Combination of crossflow ultrafiltration, monolithic affinity filtration, and quantitative reverse transcriptase PCR for rapid concentration and quantification of model viruses in water.Environ Sci Technol. 2012 Sep 18;46(18):10073-80. doi: 10.1021/es302304t. Epub 2012 Sep 7. Environ Sci Technol. 2012. PMID: 22917471
-
Attachment, re-mobilization, and inactivation of bacteriophage MS2 during bank filtration following simulation of a high virus load and an extreme rain event.J Contam Hydrol. 2022 Apr;246:103960. doi: 10.1016/j.jconhyd.2022.103960. Epub 2022 Jan 13. J Contam Hydrol. 2022. PMID: 35066264
-
Reduction of bacteriophage MS2 by filtration and irradiation determined by culture and quantitative real-time RT-PCR.J Water Health. 2013 Jun;11(2):256-66. doi: 10.2166/wh.2013.204. J Water Health. 2013. PMID: 23708573
-
Size exclusion-based purification and PCR-based quantitation of MS2 bacteriophage particles for environmental applications.J Virol Methods. 2015 Mar;213:135-8. doi: 10.1016/j.jviromet.2014.11.024. Epub 2014 Dec 18. J Virol Methods. 2015. PMID: 25528201
Cited by
-
Aggregation/dispersion transitions of T4 phage triggered by environmental ion availability.J Nanobiotechnology. 2017 Apr 24;15(1):32. doi: 10.1186/s12951-017-0266-5. J Nanobiotechnology. 2017. PMID: 28438164 Free PMC article.
-
Standoff detection of biological agents using laser induced fluorescence-a comparison of 294 nm and 355 nm excitation wavelengths.Biomed Opt Express. 2012 Nov 1;3(11):2964-75. doi: 10.1364/BOE.3.002964. Epub 2012 Oct 24. Biomed Opt Express. 2012. PMID: 23162732 Free PMC article.
-
Soil pH, Calcium Content and Bacteria as Major Factors Responsible for the Distribution of the Known Fraction of the DNA Bacteriophage Populations in Soils of Luxembourg.Microorganisms. 2022 Jul 19;10(7):1458. doi: 10.3390/microorganisms10071458. Microorganisms. 2022. PMID: 35889177 Free PMC article.
-
Evaluation of Virus Reduction by Ultrafiltration with Coagulation-Sedimentation in Water Reclamation.Food Environ Virol. 2017 Dec;9(4):453-463. doi: 10.1007/s12560-017-9301-9. Epub 2017 Apr 28. Food Environ Virol. 2017. PMID: 28455611
-
Development of Stabilizing Solution for Long-Term Storage of Bacteriophages at Room Temperature and Application to Control Foodborne Pathogens.Viruses. 2024 Jul 17;16(7):1155. doi: 10.3390/v16071155. Viruses. 2024. PMID: 39066317 Free PMC article.
References
-
- Anders, R., and C. V. Chrysikopoulos. 2006. Evaluation of the factors controlling the time-dependent inactivation rate coefficients of bacteriophage MS2 and PRD1. Environ. Sci. Technol. 40:3237-3242. - PubMed
-
- Anderson, T. F., C. Rappaport, and N. A. Muscatine. 1953. On the structure and osmotic properties of phage particles. Ann. Inst. Pasteur (Paris) 84:5-14. - PubMed
-
- AWPRC Study Group on Health Related Water Microbiology. 1991. Bacteriophages as model viruses in water quality control. Water Res. 25:529-545.
-
- Borrego, J. J., and P. Romero. 1985. Coliphage survival in seawater. Water Res. 19:557-562.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

