A small-molecule inhibitor of MDMX activates p53 and induces apoptosis
- PMID: 21075910
- PMCID: PMC3058295
- DOI: 10.1158/1535-7163.MCT-10-0581
A small-molecule inhibitor of MDMX activates p53 and induces apoptosis
Abstract
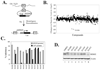
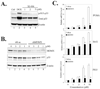
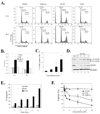
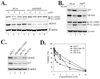
The p53 inactivation caused by aberrant expression of its major regulators (e.g., MDM2 and MDMX) contributes to the genesis of a large number of human cancers. Recent studies have shown that restoration of p53 activity by counteracting p53 repressors is a promising anticancer strategy. Although agents (e.g., nutlin-3a) that disrupt MDM2-p53 interaction can inhibit tumor growth, they are less effective in cancer cells that express high levels of MDMX. MDMX binds to p53 and can repress the tumor suppressor function of p53 through inhibiting its trans-activation activity and/or destabilizing the protein. Here we report the identification of a benzofuroxan derivative [7-(4-methylpiperazin-1-yl)-4-nitro-1-oxido-2,1,3-benzoxadiazol-1-ium, NSC207895] that could inhibit MDMX expression in cancer cells through a reporter-based drug screening. Treatments of MCF-7 cells with this small-molecule MDMX inhibitor activated p53, resulting in elevated expression of proapoptotic genes (e.g., PUMA, BAX, and PIG3). Importantly, this novel small-molecule p53 activator caused MCF-7 cells to undergo apoptosis and acted additively with nutlin-3a to activate p53 and decrease the viability of cancer cells. These results thus show that small molecules targeting MDMX expression would be of therapeutic benefits.
©2010 AACR.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures


Similar articles
-
A small-molecule p53 activator induces apoptosis through inhibiting MDMX expression in breast cancer cells.Neoplasia. 2011 Jul;13(7):611-9. doi: 10.1593/neo.11438. Neoplasia. 2011. PMID: 21750655 Free PMC article.
-
Induction of p53 expression and apoptosis by a recombinant dual-target MDM2/MDMX inhibitory protein in wild-type p53 breast cancer cells.Int J Oncol. 2013 Dec;43(6):1935-42. doi: 10.3892/ijo.2013.2138. Epub 2013 Oct 14. Int J Oncol. 2013. PMID: 24126697
-
Blockade of Hsp90 by 17AAG antagonizes MDMX and synergizes with Nutlin to induce p53-mediated apoptosis in solid tumors.Cell Death Dis. 2011 May 12;2(5):e156. doi: 10.1038/cddis.2011.39. Cell Death Dis. 2011. PMID: 21562588 Free PMC article.
-
Medicinal Chemistry Strategies to Disrupt the p53-MDM2/MDMX Interaction.Med Res Rev. 2016 Sep;36(5):789-844. doi: 10.1002/med.21393. Epub 2016 Jun 15. Med Res Rev. 2016. PMID: 27302609 Review.
-
[NEGATIVE REGULATORS OF TUMOR SUPPRESSOR P53 IN THE CONTEXT OF ANTICANCER THERAPY].Tsitologiia. 2015;57(12):847-54. Tsitologiia. 2015. PMID: 26995961 Review. Russian.
Cited by
-
Genome-wide characterization of pancreatic adenocarcinoma patients using next generation sequencing.PLoS One. 2012;7(10):e43192. doi: 10.1371/journal.pone.0043192. Epub 2012 Oct 10. PLoS One. 2012. PMID: 23071490 Free PMC article.
-
Targeting Mdmx to treat breast cancers with wild-type p53.Cell Death Dis. 2015 Jul 16;6(7):e1821. doi: 10.1038/cddis.2015.173. Cell Death Dis. 2015. PMID: 26181202 Free PMC article.
-
It's Getting Complicated-A Fresh Look at p53-MDM2-ARF Triangle in Tumorigenesis and Cancer Therapy.Front Cell Dev Biol. 2022 Jan 26;10:818744. doi: 10.3389/fcell.2022.818744. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35155432 Free PMC article. Review.
-
The emerging roles of E3 ubiquitin ligases in ovarian cancer chemoresistance.Cancer Drug Resist. 2021 Jun 19;4(2):365-381. doi: 10.20517/cdr.2020.115. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582023 Free PMC article. Review.
-
Emerging therapies targeting the ubiquitin proteasome system in cancer.J Clin Invest. 2014 Jan;124(1):6-12. doi: 10.1172/JCI71602. Epub 2014 Jan 2. J Clin Invest. 2014. PMID: 24382383 Free PMC article.
References
-
- Toledo F, Wahl G. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. - PubMed
-
- Vousden K, Prives C. Blinded by the light: The growing complexicty of p53. Cell. 2009;137:413–431. - PubMed
-
- Oliner J, Pietenpol J, Thiagalingam S, Gyuris J, Kinzler K, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. - PubMed
-
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. - PubMed
-
- Kubbutat M, Jones S, Vousden K. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

