The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis
- PMID: 21075971
- PMCID: PMC3764840
- DOI: 10.1177/1073858410381531
The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis
Abstract
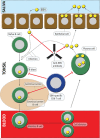
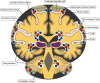
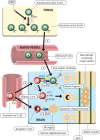
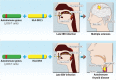
There is increasing evidence that infection with the Epstein-Barr virus (EBV) plays a role in the development of multiple sclerosis (MS), a chronic inflammatory demyelinating disease of the CNS. This article provides a four-tier hypothesis proposing (1) EBV infection is essential for the development of MS; (2) EBV causes MS in genetically susceptible individuals by infecting autoreactive B cells, which seed the CNS where they produce pathogenic autoantibodies and provide costimulatory survival signals to autoreactive T cells that would otherwise die in the CNS by apoptosis; (3) the susceptibility to develop MS after EBV infection is dependent on a genetically determined quantitative deficiency of the cytotoxic CD8+ T cells that normally keep EBV infection under tight control; and (4) sunlight and vitamin D protect against MS by increasing the number of CD8+ T cells available to control EBV infection. The hypothesis makes predictions that can be tested, including the prevention and successful treatment of MS by controlling EBV infection.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures

References
-
- Acheson ED, Bachrach CA, Wright FM. 1960. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl 35:132–47 - PubMed
-
- Amadori A, Zamarchi R, De Silvestro G, Forza G, Cavatton G, Danieli GA. and others. 1995. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat Med 1:1279–83 - PubMed
-
- Antel JP, Peeples DM, Reder AT, Arnason BGW. 1984. Analysis of T regulator cell surface markers and functional properties in multiple sclerosis. J Neuroimmunol 6:93–103 - PubMed
-
- Armengol MP, Juan M, Lucas-Martín A, Fernández-Figueras MT, Jaraquemada D, Gallart T. and others. 2001. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol 159:861–73 - PMC - PubMed
-
- Ascherio A, Munger KL. 2007. Environmental risk factors for multiple sclerosis: Part I. The role of infection. Ann Neurol 61:288–99 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

