Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells
- PMID: 21075976
- PMCID: PMC3033965
- DOI: 10.1158/1940-6207.CAPR-10-0224
Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells
Abstract
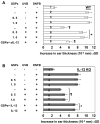
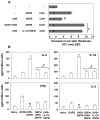
The inhibition of UVB-induced immunosuppression by dietary grape seed proanthocyanidins (GSP) has been associated with the induction of interleukin (IL)-12 in mice, and we now confirm that GSPs do not inhibit UVB-induced immunosuppression in IL-12p40 knockout (IL-12 KO) mice and that treatment of these mice with recombinant IL-12 restores the inhibitory effect. To characterize the cell population responsible for the GSP-mediated inhibition of UVB-induced immunosuppression and the role of IL-12 in this process, we used an adoptive transfer approach. Splenocytes and draining lymph nodes were harvested from mice that had been administered dietary GSPs (0.5%-1.0%, w/w), exposed to UVB, and sensitized by the application of 2,4-dinitrofluorobenzene (DNFB) onto the UVB-exposed skin. CD8(+) and CD4(+) T cells were positively selected and transferred into naive mice that were subsequently challenged by application of DNFB on the ear skin. Naive recipients that received CD8(+) T cells from GSP-treated, UVB-irradiated donors exhibited full contact hypersensitivity (CHS) response. Naive mice that received CD4(+) suppressor T cells from GSP-treated, UVB-exposed mice could mount a CHS response after sensitization and subsequent challenge with DNFB. On culture, the CD8(+) T cells from GSP-treated, UVB-exposed mice secreted higher levels (5- to 8-fold) of Th1 cytokines than CD8(+) T cells from UVB-irradiated mice not treated with GSPs. CD4(+) T cells from GSP-treated, UVB-exposed mice secreted significantly lower levels (80%-100%) of Th2 cytokines than CD4(+) T cells from UVB-exposed mice not treated with GSPs. These data suggest that GSPs inhibit UVB-induced immunosuppression by stimulating CD8(+) effector T cells and diminishing regulatory CD4(+) T cells.
©2010 AACR.
Conflict of interest statement
Figures


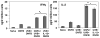
Similar articles
-
Proanthocyanidins from grape seeds inhibit UV-radiation-induced immune suppression in mice: detection and analysis of molecular and cellular targets.Photochem Photobiol. 2015 Jan-Feb;91(1):156-62. doi: 10.1111/php.12330. Epub 2014 Sep 8. Photochem Photobiol. 2015. PMID: 25112437 Free PMC article. Review.
-
Bioactive grape proanthocyanidins enhance immune reactivity in UV-irradiated skin through functional activation of dendritic cells in mice.Cancer Prev Res (Phila). 2013 Mar;6(3):242-52. doi: 10.1158/1940-6207.CAPR-12-0320. Epub 2013 Jan 15. Cancer Prev Res (Phila). 2013. PMID: 23321928 Free PMC article.
-
Dietary grape seed proanthocyanidins inactivate regulatory T cells by promoting NER-dependent DNA repair in dendritic cells in UVB-exposed skin.Oncotarget. 2017 Jul 25;8(30):49625-49636. doi: 10.18632/oncotarget.17867. Oncotarget. 2017. PMID: 28548949 Free PMC article.
-
Silymarin inhibits ultraviolet radiation-induced immune suppression through DNA repair-dependent activation of dendritic cells and stimulation of effector T cells.Biochem Pharmacol. 2013 Apr 15;85(8):1066-76. doi: 10.1016/j.bcp.2013.01.026. Epub 2013 Feb 5. Biochem Pharmacol. 2013. PMID: 23395695 Free PMC article.
-
Interleukin 12 breaks ultraviolet light induced immunosuppression by affecting CD8+ rather than CD4+ T cells.J Invest Dermatol. 1998 Mar;110(3):272-6. doi: 10.1046/j.1523-1747.1998.00111.x. J Invest Dermatol. 1998. PMID: 9506448 Review.
Cited by
-
Proanthocyanidins from grape seeds inhibit UV-radiation-induced immune suppression in mice: detection and analysis of molecular and cellular targets.Photochem Photobiol. 2015 Jan-Feb;91(1):156-62. doi: 10.1111/php.12330. Epub 2014 Sep 8. Photochem Photobiol. 2015. PMID: 25112437 Free PMC article. Review.
-
Dietary proanthocyanidins inhibit UV radiation-induced skin tumor development through functional activation of the immune system.Mol Nutr Food Res. 2016 Jun;60(6):1374-82. doi: 10.1002/mnfr.201501026. Epub 2016 Apr 13. Mol Nutr Food Res. 2016. PMID: 26991736 Free PMC article. Review.
-
Dietary agents in cancer prevention: an immunological perspective.Photochem Photobiol. 2012 Sep-Oct;88(5):1083-98. doi: 10.1111/j.1751-1097.2012.01128.x. Epub 2012 Mar 30. Photochem Photobiol. 2012. PMID: 22372381 Free PMC article. Review.
-
Grape seeds: ripe for cancer chemoprevention.Cancer Prev Res (Phila). 2013 Jul;6(7):617-21. doi: 10.1158/1940-6207.CAPR-13-0193. Epub 2013 Jun 14. Cancer Prev Res (Phila). 2013. PMID: 23771521 Free PMC article.
-
Grape seed proanthocyanidins improve lymphatic drainage and blood perfusion in secondary lymphedema models.Front Oncol. 2025 Jun 6;15:1553090. doi: 10.3389/fonc.2025.1553090. eCollection 2025. Front Oncol. 2025. PMID: 40548114 Free PMC article.
References
-
- Toews GB, Bergstresser PR, Streilein JW, Sullivan S. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53. - PubMed
-
- Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. - PubMed
-
- Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

