The N-terminal domain of the Flo1 flocculation protein from Saccharomyces cerevisiae binds specifically to mannose carbohydrates
- PMID: 21076009
- PMCID: PMC3019810
- DOI: 10.1128/EC.00185-10
The N-terminal domain of the Flo1 flocculation protein from Saccharomyces cerevisiae binds specifically to mannose carbohydrates
Abstract
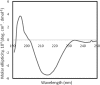
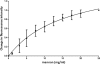
Saccharomyces cerevisiae cells possess a remarkable capacity to adhere to other yeast cells, which is called flocculation. Flocculation is defined as the phenomenon wherein yeast cells adhere in clumps and sediment rapidly from the medium in which they are suspended. These cell-cell interactions are mediated by a class of specific cell wall proteins, called flocculins, that stick out of the cell walls of flocculent cells. The N-terminal part of the three-domain protein is responsible for carbohydrate binding. We studied the N-terminal domain of the Flo1 protein (N-Flo1p), which is the most important flocculin responsible for flocculation of yeast cells. It was shown that this domain is both O and N glycosylated and is structurally composed mainly of β-sheets. The binding of N-Flo1p to D-mannose, α-methyl-D-mannoside, various dimannoses, and mannan confirmed that the N-terminal domain of Flo1p is indeed responsible for the sugar-binding activity of the protein. Moreover, fluorescence spectroscopy data suggest that N-Flo1p contains two mannose carbohydrate binding sites with different affinities. The carbohydrate dissociation constants show that the affinity of N-Flo1p for mono- and dimannoses is in the millimolar range for the binding site with low affinity and in the micromolar range for the binding site with high affinity. The high-affinity binding site has a higher affinity for low-molecular-weight (low-MW) mannose carbohydrates and no affinity for mannan. However, mannan as well as low-MW mannose carbohydrates can bind to the low-affinity binding site. These results extend the cellular flocculation model on the molecular level.
Figures




References
-
- Andrade M. A., Chacon P., Merelo J. J., Moran F. 1993. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 6:383–390 - PubMed
-
- Benoit R. M., Wilhelm R. N., Scherer-Becker D., Ostermeier C. 2006. An improved method for fast, robust, and seamless integration of DNA fragments into multiple plasmids. Protein Expr. Purif. 45:66–71 - PubMed
-
- Caro L. H., et al. 1997. In silico identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13:1477–1489 - PubMed
-
- Dean N. 1999. Asparagine-linked glycosylation in the yeast Golgi. Biochim. Biophys. Acta 1426:309–322 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

