Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis
- PMID: 21076333
- PMCID: PMC4167405
- DOI: 10.1097/WNP.0b013e3181fe0709
Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis
Abstract
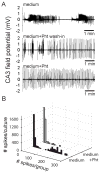
In organotypic hippocampal slice cultures, principal neurons form aberrant excitatory connections with other principal cells in response to slicing induced deafferentation, similar to mechanisms underlying epileptogenesis in posttraumatic epilepsy. To investigate the consequences of this synaptogenesis, the authors recorded field-potential activity from area CA3 during perfusion with the complete growth medium used during incubation. At 7 days in vitro, slice cultures only displayed multiunit activity. At 14 days in vitro, the majority displayed population bursts reminiscent of interictal-like spikes, but sustained synchronous activity was rare. Band-pass filtering of interictal discharges revealed fast ripple-like complexes, similar to in vivo recordings. Spontaneous ictal-like activity became progressively more prevalent with age: at 21 days in vitro, 50% of organotypic hippocampal slice cultures displayed long-lasting, ictal-like discharges that could be suppressed by phenytoin, whereas interictal activity was not suppressed. The fraction of cultures displaying ictal events continually increased with incubation time. Quantification of population spike activity throughout epileptogenesis using automatic detection and clustering algorithms confirmed the appearance of interictal-like activity before ictal-like discharges and also revealed high-frequency pathologic multiunit activity in slice cultures at 14 to 17 days in vitro. These experiments indicate that interictal-like spikes precede the appearance of ictal-like activity in a reduced in vitro preparation. Epileptiform activity in cultures resembled in vivo epilepsy, including sensitivity to anticonvulsants and steadily increasing seizure incidence over time, although seizure frequency and rate of epileptogenesis were higher in vitro. Organotypic hippocampal slice cultures comprise a useful model system for investigating mechanisms of epileptogenesis as well as developing antiepileptic and antiepileptogenic drugs.
Figures






References
-
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. - PubMed
-
- Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J Neurophysiol. 2004;92:3582–3595. - PubMed
-
- Behrens CJ, van den Boom LP, de HL, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

