24 weeks of valganciclovir prophylaxis in children after renal transplantation: a 4-year experience
- PMID: 21076375
- PMCID: PMC3682771
- DOI: 10.1097/TP.0b013e3181ffffd3
24 weeks of valganciclovir prophylaxis in children after renal transplantation: a 4-year experience
Abstract
Background: Cytomegalovirus (CMV) is the most common opportunistic infection after solid-organ transplant. Valganciclovir prophylaxis significantly reduces disease, but limited data are available on its use in children. Recently, an increase in delayed-onset CMV disease has been noted with some arguing that longer prophylaxis may decrease late-onset disease.
Methods: Single-center, retrospective analysis of pediatric renal transplant patients receiving 24 weeks valganciclovir prophylaxis (15 mg/kg/day, maximum 900 mg/day) from January 2004 to December 2008, aiming to measure the incidence of CMV disease and toxicity of valganciclovir.
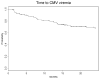
Results: We enrolled 111 patients, 60% males, 46% African Americans, and median age at transplant 14.5 years (range 1.4-20.4 years). Sixty-nine percent of donors and 44% of recipients were seropositive pretransplant. Median duration of valganciclovir use was 5.9 months (range 0.5-24 months). CMV viremia and disease occurred in 27% and 4.5%, respectively. All patients with disease presented after prophylaxis ended and all were D+/R-. Thymoglobulin use (P = 0.04) and positive donor CMV status (P = 0.02) were associated with a higher risk of CMV viremia. Twenty-four percent had hematologic toxicity directly associated with valganciclovir.
Conclusions: Valganciclovir use in children was effective as prophylaxis against CMV disease; no children at our institution developed disease while on therapy. Our regimen of 24 weeks of prophylaxis was associated with a lower rate of late-onset disease than previous reports with 12-week regimens. Further controlled studies should be considered to compare longer versus shorter periods of prophylaxis and dose reductions and their impact on prevention of late-onset disease, resistance, cost, and toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hodson EM, Craig JC, Strippoli GF, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008:CD003774. - PubMed
-
- Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611. - PubMed
-
- Singh N. Antiviral drugs for cytomegalovirus in transplant recipients: Advantages of preemptive therapy. Rev Med Virol. 2006;16:281. - PubMed
-
- Snydman DR. The case for cytomegalovirus prophylaxis in solid organ transplantation. Rev Med Virol. 2006;16:289. - PubMed
-
- Mwintshi K, Brennan DC. Prevention and management of cytomegalovirus infection in solid-organ transplantation. Expert Rev Anti Infect Ther. 2007;5:295. - PubMed