The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis
- PMID: 21076392
- PMCID: PMC3018791
- DOI: 10.1038/emboj.2010.280
The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis
Abstract
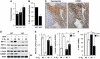
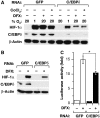
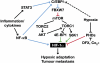
Inflammation and hypoxia are known to promote the metastatic progression of tumours. The CCAAT/enhancer-binding protein-δ (C/EBPδ, CEBPD) is an inflammatory response gene and candidate tumour suppressor, but its physiological role in tumourigenesis in vivo is unknown. Here, we demonstrate a tumour suppressor function of C/EBPδ using transgenic mice overexpressing the Neu/Her2/ERBB2 proto-oncogene in the mammary gland. Unexpectedly, this study also revealed that C/EBPδ is necessary for efficient tumour metastasis. We show that C/EBPδ is induced by hypoxia in tumours in vivo and in breast tumour cells in vitro, and that C/EBPδ-deficient cells exhibit reduced glycolytic metabolism and cell viability under hypoxia. C/EBPδ supports CXCR4 expression. On the other hand, C/EBPδ directly inhibits expression of the tumour suppressor F-box and WD repeat-domain containing 7 gene (FBXW7, FBW7, AGO, Cdc4), encoding an F-box protein that promotes degradation of the mammalian target of rapamycin (mTOR). Consequently, C/EBPδ enhances mTOR/AKT/S6K1 signalling and augments translation and activity of hypoxia-inducible factor-1α (HIF-1α), which is necessary for hypoxia adaptation. This work provides new insight into the mechanisms by which metastasis-promoting signals are induced specifically under hypoxia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


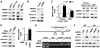
Comment in
-
C/EBPδ: friend or foe? a novel role for C/EBPδ in metastasis.EMBO J. 2010 Dec 15;29(24):4063-5. doi: 10.1038/emboj.2010.308. EMBO J. 2010. PMID: 21157479 Free PMC article.
References
-
- Abraham RT (2004) mTOR as a positive regulator of tumor cell responses to hypoxia. Curr Top Microbiol Immunol 279: 299–319 - PubMed
-
- Agrawal S, Hofmann WK, Tidow N, Ehrich M, van den Boom D, Koschmieder S, Berdel WE, Serve H, Muller-Tidow C (2007) The C/EBPdelta tumor suppressor is silenced by hypermethylation in acute myeloid leukemia. Blood 109: 3895–3905 - PubMed
-
- Barresi V, Vitarelli E, Cerasoli S, Barresi G (2009) The cell growth inhibitory transcription factor C/EBPdelta is expressed in human meningiomas in association with low histological grade and proliferation index. J Neurooncol 97: 233–240 - PubMed
-
- Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, Johnson RS, Bergers G (2003) The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4: 133–146 - PubMed
-
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E (1998) Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394: 485–490 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

