BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping
- PMID: 21076401
- PMCID: PMC2998174
- DOI: 10.1038/nsmb.1943
BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping
Abstract
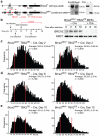
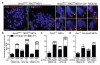
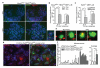
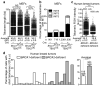
The tumor suppressor protein BRCA2 is a key component of the homologous recombination pathway of DNA repair, acting as the loader of RAD51 recombinase at sites of double-strand breaks. Here we show that BRCA2 associates with telomeres during the S and G2 phases of the cell cycle and facilitates the loading of RAD51 onto telomeres. Conditional deletion of Brca2 and inhibition of Rad51 in mouse embryonic fibroblasts (MEFs), but not inactivation of Brca1, led to shortening of telomeres and accumulation of fragmented telomeric signals--a hallmark of telomere fragility that is associated with replication defects. These findings suggest that BRCA2-mediated homologous recombination reactions contribute to the maintenance of telomere length by facilitating telomere replication and imply that BRCA2 has an essential role in maintaining telomere integrity during unchallenged cell proliferation. Mouse mammary tumors that lacked Brca2 accumulated telomere dysfunction-induced foci. Human breast tumors in which BRCA2 was mutated had shorter telomeres than those in which BRCA1 was mutated, suggesting that the genomic instability in BRCA2-deficient tumors was due in part to telomere dysfunction.
Figures



References
-
- Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer. 2004;4:665–676. - PubMed
-
- Evers B, Jonkers J. Mouse models of BRCA1 and BRCA2 deficiency: past lessons, current understanding and future prospects. Oncogene. 2006;25:5885–5897. - PubMed
-
- Thorslund T, West SC. BRCA2: a universal recombinase regulator. Oncogene. 2007;26:7720–7730. - PubMed
-
- Venkitaraman AR. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu. Rev. Pathol. 2009;4:461–487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

