Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies
- PMID: 21076402
- PMCID: PMC2997185
- DOI: 10.1038/nsmb.1950
Distinct conformational states of HIV-1 gp41 are recognized by neutralizing and non-neutralizing antibodies
Abstract
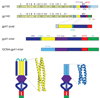
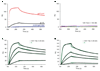
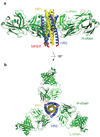
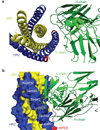
HIV-1 envelope glycoprotein gp41 undergoes large conformational changes to drive fusion of viral and target cell membranes, adopting at least three distinct conformations during the viral entry process. Neutralizing antibodies against gp41 block HIV-1 infection by targeting gp41's membrane-proximal external region in a fusion-intermediate state. Here we report biochemical and structural evidence that non-neutralizing antibodies, capable of binding with high affinity to an immunodominant segment adjacent to the neutralizing epitopes in the membrane-proximal region, recognize a gp41 conformation that exists only when membrane fusion is complete. We propose that these non-neutralizing antibodies are induced in HIV-1-infected individuals by gp41 in a triggered, postfusion form and contribute to production of ineffective humoral responses. These results have important implications for gp41-based vaccine design.
Figures

Similar articles
-
Binding of HIV-1 gp41-directed neutralizing and non-neutralizing fragment antibody binding domain (Fab) and single chain variable fragment (ScFv) antibodies to the ectodomain of gp41 in the pre-hairpin and six-helix bundle conformations.PLoS One. 2014 Aug 8;9(8):e104683. doi: 10.1371/journal.pone.0104683. eCollection 2014. PLoS One. 2014. PMID: 25105806 Free PMC article.
-
Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region.Nat Struct Mol Biol. 2010 Dec;17(12):1492-4. doi: 10.1038/nsmb.1944. Epub 2010 Nov 14. Nat Struct Mol Biol. 2010. PMID: 21076400 Free PMC article.
-
Sequestering of the prehairpin intermediate of gp41 by peptide N36Mut(e,g) potentiates the human immunodeficiency virus type 1 neutralizing activity of monoclonal antibodies directed against the N-terminal helical repeat of gp41.J Virol. 2008 Oct;82(20):10032-41. doi: 10.1128/JVI.01050-08. Epub 2008 Jul 30. J Virol. 2008. PMID: 18667502 Free PMC article.
-
Neutralizing Antibodies Targeting HIV-1 gp41.Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review.
-
Progress towards the development of a HIV-1 gp41-directed vaccine.Curr HIV Res. 2004 Apr;2(2):193-204. doi: 10.2174/1570162043484933. Curr HIV Res. 2004. PMID: 15078183 Review.
Cited by
-
Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes.J Virol. 2011 Aug;85(15):7788-96. doi: 10.1128/JVI.00555-11. Epub 2011 May 25. J Virol. 2011. PMID: 21613394 Free PMC article.
-
Spatiotemporal hierarchy in antibody recognition against transmitted HIV-1 envelope glycoprotein during natural infection.Retrovirology. 2016 Feb 17;13:12. doi: 10.1186/s12977-016-0243-3. Retrovirology. 2016. PMID: 26883323 Free PMC article.
-
Synthetic Fab fragments that bind the HIV-1 gp41 heptad repeat regions.Biochem Biophys Res Commun. 2011 Oct 7;413(4):611-5. doi: 10.1016/j.bbrc.2011.09.012. Epub 2011 Sep 6. Biochem Biophys Res Commun. 2011. PMID: 21925149 Free PMC article.
-
Human immunoglobulin E flexes between acutely bent and extended conformations.Nat Struct Mol Biol. 2014 Apr;21(4):397-404. doi: 10.1038/nsmb.2795. Epub 2014 Mar 16. Nat Struct Mol Biol. 2014. PMID: 24632569 Free PMC article.
-
HIV-1 fusion inhibitors targeting the membrane-proximal external region of Env spikes.Nat Chem Biol. 2020 May;16(5):529-537. doi: 10.1038/s41589-020-0496-y. Epub 2020 Mar 9. Nat Chem Biol. 2020. PMID: 32152540 Free PMC article.
References
-
- Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. - PubMed
-
- Allan JS, et al. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985;228:1091–1094. - PubMed
-
- Veronese FD, et al. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985;229:1402–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

