Neuroprotection by the Traditional Chinese Medicine, Tao-Hong-Si-Wu-Tang, against Middle Cerebral Artery Occlusion-Induced Cerebral Ischemia in Rats
- PMID: 21076527
- PMCID: PMC2975073
- DOI: 10.1155/2011/803015
Neuroprotection by the Traditional Chinese Medicine, Tao-Hong-Si-Wu-Tang, against Middle Cerebral Artery Occlusion-Induced Cerebral Ischemia in Rats
Abstract
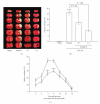
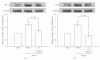
Tao-Hong-Si-Wu-Tang (THSWT) is a famous traditional Chinese medicine (TMC). In the present study, oral administration of THSWT (0.7 and 1.4 g kg(-1)day(-1)) for 14 days before MCAO dose-dependently attenuated focal cerebral ischemia in rats. MCAO-induced focal cerebral ischemia was associated with increases in hypoxia-inducible factor (HIF)-1α, inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α, and active caspase-3 expressions in ischemic regions. These expressions were obviously inhibited by 0.7 g kg(-1)day(-1) THSWT treatment. In addition, THSWT inhibited platelet aggregation stimulated by collagen in washed platelets. In an in vivo study, THSWT (16 g kg(-1)) significantly prolonged platelet plug formation in mice. However, THSWT (20 and 40 μg mL(-1)) did not significantly reduce the electron spin resonance (ESR) signal intensity of hydroxyl radical (OH(•)) formation. In conclusion, the most important findings of this study demonstrate for the first time that THSWT possesses potent neuroprotective activity against MCAO-induced focal cerebral ischemia in vivo. This effect may be mediated, at least in part, by the inhibition of both HIF-1α and TNF-α activation, followed by the inhibition of inflammatory responses (i.e., iNOS expression), apoptosis formation (active caspase-3), and platelet activation, resulting in a reduction in the infarct volume in ischemia-reperfusion brain injury.
Figures
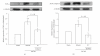



References
-
- Kuroda S, Siesjö BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clinical Neuroscience. 1997;4(4):199–212. - PubMed
-
- Abumiya T, Fitridge R, Mazur C, et al. Integrin α(IIb)β3 inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31(6):1402–1410. - PubMed
-
- Iadecola C, Zhang F, Casey R, Clark HB, Ross ME. Inducible nitric oxide synthase gene expression in vascular cells after transient focal cerebral ischemia. Stroke. 1996;27(8):1373–1380. - PubMed
-
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. Journal of Cerebral Blood Flow and Metabolism. 2001;21(1):2–14. - PubMed
-
- Lipton P. Ischemic cell death in brain neurons. Physiological Reviews. 1999;79(4):1431–1568. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

