The role of HDAC6 in cancer
- PMID: 21076528
- PMCID: PMC2975074
- DOI: 10.1155/2011/875824
The role of HDAC6 in cancer
Abstract
Histone deacetylase 6 (HDAC6), a member of the HDAC family whose major substrate is α-tubulin, has become a target for drug development to treat cancer due to its major contribution in oncogenic cell transformation. Overexpression of HDAC6 correlates with tumorigenesis and improved survival; therefore, HDAC6 may be used as a marker for prognosis. Previous work demonstrated that in multiple myeloma cells, inhibition of HDAC6 results in apoptosis. Furthermore, HDAC6 is required for the activation of heat-shock factor 1 (HSF1), an activator of heat-shock protein encoding genes (HSPs) and CYLD, a cylindromatosis tumor suppressor gene. HDAC6 contributes to cancer metastasis since its upregulation increases cell motility in breast cancer MCF-7 cells and its interaction with cortactin regulates motility. HDAC6 also affects transcription and translation by regulating the heat-shock protein 90 (Hsp90) and stress granules (SGs), respectively. This review will discuss the role of HDAC6 in the pathogenesis and treatment of cancer.
Figures


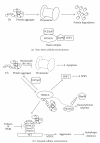
References
-
- Marks PA, Miller T, Richon VM. Histone deacetylases. Current Opinion in Pharmacology. 2003;3:344–351. - PubMed
-
- Saji S, Kawakami M, Hayashi S-I, et al. Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor-positive breast cancer. Oncogene. 2005;24(28):4531–4539. - PubMed
-
- Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Letters. 2008;269(1):7–17. - PubMed
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer Journal for Clinicians. 2008;58(2):71–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

