Perspectives on Regulatory T Cell Therapies
- PMID: 21076548
- PMCID: PMC2969127
- DOI: 10.1159/000235929
Perspectives on Regulatory T Cell Therapies
Abstract
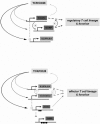
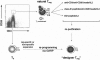
Adoptive transfer in animal models clearly indicate an essential role of CD4+ CD25+ FOXP3+ regulatory T (T(reg)) cells in prevention and treatment of autoimmune and graft-versus-host disease. Thus, T(reg) cell therapies and development of drugs that specifically enhance T(reg) cell function and development represent promising tools to establish dominant tolerance. So far, lack of specific markers to differentiate human T(reg) cells from activated CD4+ CD25+ effector T cells, which also express FOXP3 at different levels, hampered such an approach. Recent identification of the orphan receptor glycoprotein-A repetitions predominant (GARP or LRRC32) as T(reg) cell-specific key molecule that dominantly controls FOXP3 via a positive feedback loop opens up new perspectives for molecular and cellular therapies. This brief review focuses on the role of GARP as a safeguard of a complex regulatory network of human T(reg) cells and its implications for regulatory T cell therapies in autoimmunity and graft-versus-host disease.
Tierexperimentelle Arbeiten zur adoptiven T-Zell-Therapie zeigten eindrücklich die bedeutende Funktion von CD4+ CD25+ FOXP3+ regulatorischen T(Treg)-Zellen in der Prävention und Behandlung von Autoimmunkrankheiten und der Graft-versus-host-Erkrankung. Hierdurch stellt die Anwendung einer adoptiven Treg-Zelltherapie bzw. die Entwicklung von Medikamenten, die die Funktion von Treg-Zellen spezifisch verstärken, eine neue Perspektive zur Etablierung einer dominanten Toleranz dar. Bisher wurde diese Entwicklung durch den Mangel spezifischer Oberflächenmarker zur sicheren Unterscheidung von Treg-Zellen und aktivierten CD4+ CD25+ Effektor-T-Zellen, die ebenfalls FOXP3 in unterschiedlichem Maße exprimieren, gehemmt. Die Entdeckung des Rezeptors «glycoprotein-A repetitions predominant» (GARP bzw. LRRC32) als Treg-Zell-spezifisches Schlüsselmolekül, das dominant FOXP3 in Verbindung mit einem positiven «feedback loop» kontrolliert, eröffnet neue Perspektiven für die zelluläre und molekulare Treg-Zell-Therapie. Diese Übersicht befasst sich mit den Perspektiven, die die Entdeckung von GARP als «safeguard» eines komplexen regulatorischen Netzwerks humaner Treg-Zellen mit sich bringen, sowie mit deren Bedeutung für regulatorische T-Zell-Therapien bei Autoimmunkrankheiten and der Graft-versus-host-Erkrankung.
Figures
References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Ochs HD, Ziegler SF, Torgerson TR. FOXP3 acts as a rheostat of the immune response. Immunol Rev. 2005;203:156–164. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

