An investigation of the distal histidyl hydrogen bonds in oxyhemoglobin: effects of temperature, pH, and inositol hexaphosphate
- PMID: 21077639
- PMCID: PMC3033742
- DOI: 10.1021/bi100927p
An investigation of the distal histidyl hydrogen bonds in oxyhemoglobin: effects of temperature, pH, and inositol hexaphosphate
Abstract
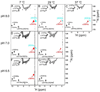
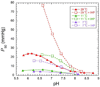
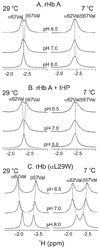
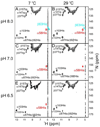
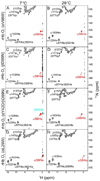
On the basis of X-ray crystal structures and electron paramagnetic resonance (EPR) measurements, it has been inferred that the O(2) binding to hemoglobin is stabilized by the hydrogen bonds between the oxygen ligands and the distal histidines. Our previous study by multinuclear nuclear magnetic resonance (NMR) spectroscopy has provided the first direct evidence of such H-bonds in human normal adult oxyhemoglobin (HbO(2) A) in solution. Here, the NMR spectra of uniformly (15)N-labeled recombinant human Hb A (rHb A) and five mutant rHbs in the oxy form have been studied under various experimental conditions of pH and temperature and also in the presence of an organic phosphate, inositol hexaphosphate (IHP). We have found significant effects of pH and temperature on the strength of the H-bond markers, i.e., the cross-peaks for the side chains of the two distal histidyl residues, α58His and β63His, which form H-bonds with the O(2) ligands. At lower pH and/or higher temperature, the side chains of the distal histidines appear to be more mobile, and the exchange with water molecules in the distal heme pockets is faster. These changes in the stability of the H-bonds with pH and temperature are consistent with the changes in the O(2) affinity of Hb as a function of pH and temperature and are clearly illustrated by our NMR experiments. Our NMR results have also confirmed that this H-bond in the β-chain is weaker than that in the α-chain and is more sensitive to changes in pH and temperature. IHP has only a minor effect on these H-bond markers compared to the effects of pH and temperature. These H-bonds are sensitive to mutations in the distal heme pockets but not affected directly by the mutations in the quaternary interfaces, i.e., α(1)β(1) and/or α(1)β(2) subunit interface. These findings provide new insights regarding the roles of temperature, hydrogen ion, and organic phosphate in modulating the structure and function of hemoglobin in solution.
Figures


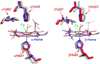
Similar articles
-
T-quaternary structure of oxy human adult hemoglobin in the presence of two allosteric effectors, L35 and IHP.Biochim Biophys Acta. 2011 Oct;1807(10):1253-61. doi: 10.1016/j.bbabio.2011.06.004. Epub 2011 Jun 15. Biochim Biophys Acta. 2011. PMID: 21703224
-
An additional H-bond in the alpha 1 beta 2 interface as the structural basis for the low oxygen affinity and high cooperativity of a novel recombinant hemoglobin (beta L105W).Biochemistry. 2000 Nov 14;39(45):13708-18. doi: 10.1021/bi001115i. Biochemistry. 2000. PMID: 11076510
-
NMR reveals hydrogen bonds between oxygen and distal histidines in oxyhemoglobin.Proc Natl Acad Sci U S A. 2000 Sep 12;97(19):10354-8. doi: 10.1073/pnas.190254697. Proc Natl Acad Sci U S A. 2000. PMID: 10962034 Free PMC article.
-
Ligand binding properties and structural studies of recombinant and chemically modified hemoglobins altered at beta 93 cysteine.Biochemistry. 2002 Oct 1;41(39):11901-13. doi: 10.1021/bi0202880. Biochemistry. 2002. PMID: 12269835
-
Novel recombinant hemoglobin, rHb (beta N108Q), with low oxygen affinity, high cooperativity, and stability against autoxidation.Biochemistry. 2000 Nov 14;39(45):13719-29. doi: 10.1021/bi001116a. Biochemistry. 2000. PMID: 11076511
Cited by
-
A biochemical--biophysical study of hemoglobins from woolly mammoth, Asian elephant, and humans.Biochemistry. 2011 Aug 30;50(34):7350-60. doi: 10.1021/bi200777j. Epub 2011 Aug 2. Biochemistry. 2011. PMID: 21806075 Free PMC article.
-
Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose.PLoS Genet. 2018 Apr 2;14(4):e1007331. doi: 10.1371/journal.pgen.1007331. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29608560 Free PMC article.
-
Unlocking the binding and reaction mechanism of hydroxyurea substrates as biological nitric oxide donors.J Chem Inf Model. 2012 May 25;52(5):1288-97. doi: 10.1021/ci300035c. Epub 2012 May 9. J Chem Inf Model. 2012. PMID: 22519847 Free PMC article.
-
Autoxidation and oxygen binding properties of recombinant hemoglobins with substitutions at the αVal-62 or βVal-67 position of the distal heme pocket.J Biol Chem. 2013 Aug 30;288(35):25512-25521. doi: 10.1074/jbc.M113.474841. Epub 2013 Jul 18. J Biol Chem. 2013. PMID: 23867463 Free PMC article.
-
WAXS studies of the structural diversity of hemoglobin in solution.J Mol Biol. 2011 May 20;408(5):909-21. doi: 10.1016/j.jmb.2011.02.062. Epub 2011 Mar 21. J Mol Biol. 2011. PMID: 21420976 Free PMC article.
References
-
- Dickerson RE, Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. Menlo Park, CA: The Benjamin/Cummings Publication Co. Inc.; 1983.
-
- Olson JS, Phillips GN. Myoglobin discriminates between O2, NO, and CO by electrostatic interactions with the bound ligand. Journal of Biological Inorganic Chemistry. 1997;2:544–552.
-
- Shaanan B. Structure of human oxyhemoglobin at 2.1 Å resolution. J. Mol. Biol. 1983;171:31–59. - PubMed
-
- Park S-Y, Yokoyama T, Shibayama N, Shiro Y, Tame JRH. 1.25 Å resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J. Mol. Biol. 2006;360:690–701. - PubMed
-
- Ackers GK. Deciphering the molecular code of hemoglobin allostery. Adv. Protein Chem. 1998:185–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

