Chemoenzymatic syntheses and anti-HIV-1 activity of glucose-nucleoside conjugates as prodrugs
- PMID: 21077659
- PMCID: PMC3070195
- DOI: 10.1021/bc1002168
Chemoenzymatic syntheses and anti-HIV-1 activity of glucose-nucleoside conjugates as prodrugs
Abstract
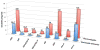
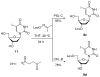
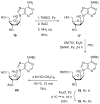
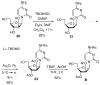
Phosphodiester linked conjugates of various nucleosides such as d4U, d4T, IdUrd, ddI, ddA, virazole, ara-A, and ara-C containing a glucosyl moiety have been described. These compounds were designed to act as prodrugs, where the corresponding 5'-monophosphates may be generated intracellularly. The synthesis of the glycoconjugates was achieved in good yields by condensation of a glucosyl phosphoramidite 7 with nucleosides in the presence of an activating agent. It was demonstrated that the glucose conjugates improve the water solubility of the nucleoside analogues, for example, up to 31-fold for the ara-A conjugate compared to that of ara-A alone. The new conjugates were tested for their anti-HIV-1 activity in human lymphocytes. These derivatives offer a convenient design for potential prodrug candidates with the possibility of improving the physicochemical properties and therapeutic activity of nucleoside analogues.
Figures





Similar articles
-
Bis(benzoyloxybenzyl)-DiPPro nucleoside diphosphates of anti-HIV active nucleoside analogues.ChemMedChem. 2015 May;10(5):891-900. doi: 10.1002/cmdc.201500063. Epub 2015 Apr 2. ChemMedChem. 2015. PMID: 25847660
-
Tri-N-Boc-tetraazamacrocycle-nucleoside conjugates: synthesis and anti-HIV activities.Nucleosides Nucleotides. 1998 May;17(5):957-68. doi: 10.1080/07328319808003466. Nucleosides Nucleotides. 1998. PMID: 9708333
-
Synthesis, anti-HIV activity, and resistance profile of thymidine phosphonomethoxy nucleosides and their bis-isopropyloxymethylcarbonyl (bisPOC) prodrugs.Bioorg Med Chem. 2007 Aug 15;15(16):5519-28. doi: 10.1016/j.bmc.2007.05.047. Epub 2007 May 25. Bioorg Med Chem. 2007. PMID: 17562366
-
The synthesis and antiviral properties of acyclic nucleoside analogues with a phosphonomethoxy fragment in the side chain.Curr Med Chem. 2006;13(24):2953-80. doi: 10.2174/092986706778521896. Curr Med Chem. 2006. PMID: 17073640 Review.
-
Strategies in the design of prodrugs of anti-HIV agents.Curr Top Med Chem. 2003;3(13):1467-95. doi: 10.2174/1568026033451763. Curr Top Med Chem. 2003. PMID: 14529522 Review.
Cited by
-
Synthesis, Structural Elucidation, and Anti-Inflammatory Activity of a Water-Soluble Derivative of Arctiin.Molecules. 2023 Feb 14;28(4):1789. doi: 10.3390/molecules28041789. Molecules. 2023. PMID: 36838775 Free PMC article.
-
The Application of Prodrugs as a Tool to Enhance the Properties of Nucleoside Reverse Transcriptase Inhibitors.Viruses. 2023 Nov 9;15(11):2234. doi: 10.3390/v15112234. Viruses. 2023. PMID: 38005911 Free PMC article. Review.
-
Chemoselective hydroxyl group transformation: an elusive target.Mol Biosyst. 2012 Oct;8(10):2484-93. doi: 10.1039/c2mb25122a. Mol Biosyst. 2012. PMID: 22695722 Free PMC article. Review.
-
Novel 1'-homo-N-2'-deoxy-α-nucleosides: synthesis, characterization and biological activity.RSC Adv. 2020;10(27):15815-15824. doi: 10.1039/D0RA03254A. Epub 2020 Apr 21. RSC Adv. 2020. PMID: 34603689 Free PMC article.
-
Synthesis, evaluation of anti-HIV-1 and anti-HCV activity of novel 2',3'-dideoxy-2',2'-difluoro-4'-azanucleosides.Bioorg Med Chem. 2012 Dec 1;20(23):6885-93. doi: 10.1016/j.bmc.2012.09.026. Epub 2012 Sep 23. Bioorg Med Chem. 2012. PMID: 23085031 Free PMC article.
References
-
- Schinazi RF, Liotta DC. Frontiers in Nucleoside and Nucleic Acids. IHL Press; Tucker, Georgia: 2004.
-
- Rachakonda S, Cartee L. Challenges in Antimicrobial Drug Discovery and the Potential of Nucleoside Antibiotics. Curr Med Chem. 2004;11:775–793. - PubMed
-
- De Clercq E. Antiviral drugs in current clinical use. J Clin Vir. 2004;30:115–133. - PubMed
-
- Chu CK. Recent Advances in Nucleosides: Chemistry and Chemotherapy. Elsevier Science; New York: 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

