Organometallic anticancer compounds
- PMID: 21077686
- PMCID: PMC3018145
- DOI: 10.1021/jm100020w
Organometallic anticancer compounds
Figures


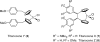

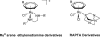

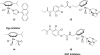
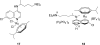
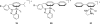
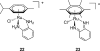
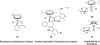
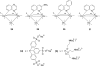



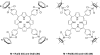
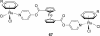
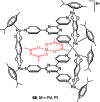
References
-
- Jakupec M. A.; Galanski M.; Arion V. B.; Hartinger C. G.; Keppler B. K. Antitumour metal compounds: more than theme and variations. Dalton Trans. 2008, 2, 183–194. - PubMed
-
- Dyson P. J.; Sava G. Metal-based antitumour drugs in the post genomic area. Dalton Trans. 2006, 1929–1933 and references therein. - PubMed
-
- Wang D.; Lippard S. J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery 2005, 4, 307–320. - PubMed
-
- Lippert B.Cisplatin, Chemistry and Biochemistry of a Leading Anticancer Drug; Verlag Helvetica Chimica Acta: Zürich, Switzerland, 1999.
-
- Hartinger C.; Dyson P. J. Bioorganometallic chemistry—from teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

