Monoclonal antibodies to B and T lymphocyte attenuator (BTLA) have no effect on in vitro B cell proliferation and act to inhibit in vitro T cell proliferation when presented in a cis, but not trans, format relative to the activating stimulus
- PMID: 21078085
- PMCID: PMC3010914
- DOI: 10.1111/j.1365-2249.2010.04259.x
Monoclonal antibodies to B and T lymphocyte attenuator (BTLA) have no effect on in vitro B cell proliferation and act to inhibit in vitro T cell proliferation when presented in a cis, but not trans, format relative to the activating stimulus
Abstract
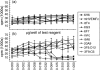
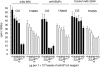
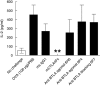
B and T lymphocyte attenuator (BTLA) is an immunoglobulin superfamily member surface protein expressed on B and T cells. Its ligand, herpesvirus entry mediator (HVEM), is believed to act as a monomeric agonist that signals via the CRD1 of HVEM to inhibit lymphocyte activation: HVEM is also the receptor for lymphotoxin-α and LIGHT, which both bind in the CRD2 and CRD3 domains of the HVEM molecule, and for CD160 which competes with BTLA. We have shown that recombinant HVEM and a panel of different monoclonal antibodies specifically bind murine BTLA on both B and T cells and that some antibodies inhibit anti-CD3ε-induced T cell proliferation in vitro, but only when constrained appropriately with a putatively cross-linking reagent. The antibodies had no significant effect on in vitro T cell proliferation in a mixed lymphocyte reaction (MLR) assay nor on in vitro DO11.10 antigen-induced T cell proliferation. None of these antibodies, nor HVEM-Fc, had any significant effect on in vitro B cell proliferation induced by anti-immunoglobulin M antibodies (±anti-CD40) or lipopolysaccharide. We further elucidated the requirements for inhibition of in vitro T cell proliferation using a beads-based system to demonstrate that the antibodies that inhibited T cell proliferation in vitro were required to be presented to the T cell in a cis, and not trans, format relative to the anti-CD3ε stimulus. We also found that antibodies that inhibited T cell proliferation in vitro had no significant effect on the antibody captured interleukin-2 associated with the in vivo activation of DO11.10 T cells transferred to syngeneic recipient BALB/c mice. These data suggest that there may be specific structural requirements for the BTLA molecule to exert its effect on lymphocyte activation and proliferation.
© 2010 Authors. Clinical and Experimental Immunology © 2010 British Society for Immunology.
Figures


References
-
- Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236–43. - PubMed
-
- Hurchla MA, Sedy JR, Drake CG, Murphy KM. B and T lymphocytle attenuator (BTLA) exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–85. - PubMed
-
- Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. Attenuating lymphocyte activity – the crystal structure of the BTLA–HVEM complex. J Biol Chem. 2005;280:39553–61. - PubMed
-
- Hurchla MA, Sedy JR, Murphy KM. Unexpected role of B and T lymphocyte attenuator in sustaining cell survival during chronic allostimulation. J Immunol. 2007;178:6073–82. - PubMed
-
- Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J Immunol. 2006;176:6603–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

