The human G93A-SOD1 mutation in a pre-symptomatic rat model of amyotrophic lateral sclerosis increases the vulnerability to a mild spinal cord compression
- PMID: 21078175
- PMCID: PMC3020590
- DOI: 10.1186/1471-2164-11-633
The human G93A-SOD1 mutation in a pre-symptomatic rat model of amyotrophic lateral sclerosis increases the vulnerability to a mild spinal cord compression
Abstract
Background: Traumatic injuries can undermine neurological functions and act as risk factors for the development of irreversible and fatal neurodegenerative disorders like amyotrophic lateral sclerosis (ALS). In this study, we have investigated how a mutation of the superoxide dismutase 1 (SOD1) gene, linked to the development of ALS, modifies the acute response to a gentle mechanical compression of the spinal cord. In a 7-day post-injury time period, we have performed a comparative ontological analysis of the gene expression profiles of injured spinal cords obtained from pre-symptomatic rats over-expressing the G93A-SOD1 gene mutation and from wild type (WT) littermates.
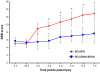
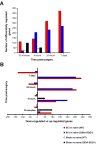
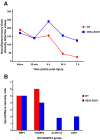
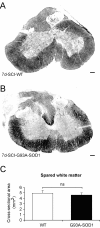
Results: The steady post-injury functional recovery observed in WT rats was accompanied by the early activation at the epicenter of injury of several growth-promoting signals and by the down-regulation of intermediate neurofilaments and of genes involved in the regulation of ion currents at the 7 day post-injury time point. The poor functional recovery observed in G93A-SOD1 transgenic animals was accompanied by the induction of fewer pro-survival signals, by an early activation of inflammatory markers, of several pro-apoptotic genes involved in cytochrome-C release and by the persistent up-regulation of the heavy neurofilament subunits and of genes involved in membrane excitability. These molecular changes occurred along with a pronounced atrophy of spinal cord motor neurones in the G93A-SOD1 rats compared to WT littermates after compression injury.
Conclusions: In an experimental paradigm of mild mechanical trauma which causes no major tissue damage, the G93A-SOD1 gene mutation alters the balance between pro-apoptotic and pro-survival molecular signals in the spinal cord tissue from the pre-symptomatic rat, leading to a premature activation of molecular pathways implicated in the natural development of ALS.
Figures





Similar articles
-
Comparative analysis of the time-dependent functional and molecular changes in spinal cord degeneration induced by the G93A SOD1 gene mutation and by mechanical compression.BMC Genomics. 2008 Oct 23;9:500. doi: 10.1186/1471-2164-9-500. BMC Genomics. 2008. PMID: 18947433 Free PMC article.
-
Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261
-
Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase.J Neuroinflammation. 2014 Mar 23;11:55. doi: 10.1186/1742-2094-11-55. J Neuroinflammation. 2014. PMID: 24655927 Free PMC article.
-
Transgenic mice in the study of ALS: the role of neurofilaments.Brain Pathol. 1998 Oct;8(4):759-69. doi: 10.1111/j.1750-3639.1998.tb00199.x. Brain Pathol. 1998. PMID: 9804382 Free PMC article. Review.
-
ALS genetic modifiers that increase survival of SOD1 mice and are suitable for therapeutic development.Prog Neurobiol. 2011 Oct;95(2):133-48. doi: 10.1016/j.pneurobio.2011.07.009. Epub 2011 Jul 26. Prog Neurobiol. 2011. PMID: 21816207 Review.
Cited by
-
Spinal cord trauma and the molecular point of no return.Mol Neurodegener. 2012 Feb 8;7:6. doi: 10.1186/1750-1326-7-6. Mol Neurodegener. 2012. PMID: 22315999 Free PMC article. Review.
-
The omega-3 fatty acid eicosapentaenoic acid accelerates disease progression in a model of amyotrophic lateral sclerosis.PLoS One. 2013 Apr 19;8(4):e61626. doi: 10.1371/journal.pone.0061626. Print 2013. PLoS One. 2013. PMID: 23620776 Free PMC article.
-
Can transcriptomics cut the gordian knot of amyotrophic lateral sclerosis?Curr Genomics. 2011 Nov;12(7):506-15. doi: 10.2174/138920211797904043. Curr Genomics. 2011. PMID: 22547957 Free PMC article.
-
Inflammation is increased with anxiety- and depression-like signs in a rat model of spinal cord injury.Brain Behav Immun. 2016 Jan;51:176-195. doi: 10.1016/j.bbi.2015.08.009. Epub 2015 Aug 18. Brain Behav Immun. 2016. PMID: 26296565 Free PMC article.
-
Type I Vs. Type II Cytokine Levels as a Function of SOD1 G93A Mouse Amyotrophic Lateral Sclerosis Disease Progression.Front Cell Neurosci. 2015 Dec 1;9:462. doi: 10.3389/fncel.2015.00462. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26648846 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

