Facultative heterochromatin formation at the IL-1 beta promoter in LPS tolerance and sepsis
- PMID: 21078560
- PMCID: PMC3021647
- DOI: 10.1016/j.cyto.2010.10.007
Facultative heterochromatin formation at the IL-1 beta promoter in LPS tolerance and sepsis
Abstract
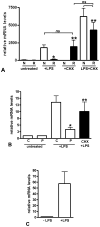
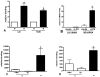
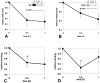
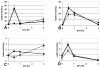
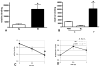
The clinical phenotype in sepsis that is observed as LPS tolerance is determined by silencing of pro-inflammatory genes like IL-1 beta (IL-1β). This study shows that facultative heterochromatin (fHC) silences IL-1β expression during sepsis, where we find dephosphorylated histone H3 serine 10 and increased binding of heterochromatin protein-1 (HP-1) to the promoter. In both human sepsis blood leukocytes and an LPS tolerant human THP-1 cell model, we show that IκBα and v-rel reticuloendotheliosis viral oncogene homolog B (RelB) function as dominant labile mediators of fHC formation at the IL-1β promoter. Protein synthesis inhibition decreases levels of IκBα and RelB, converts silent fHC to euchromatin, and restores IL-1β transcription. We further show TLR dependent NFκB p65 and histone H3 serine 10 phosphorylation binding at the promoter. We conclude that the resolution phase of sepsis, which correlates with survival in humans, may depend on the plasticity of chromatin structure as found in fHC.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Chromatin-specific remodeling by HMGB1 and linker histone H1 silences proinflammatory genes during endotoxin tolerance.Mol Cell Biol. 2009 Apr;29(7):1959-71. doi: 10.1128/MCB.01862-08. Epub 2009 Jan 21. Mol Cell Biol. 2009. PMID: 19158276 Free PMC article.
-
The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance.J Biol Chem. 2009 Oct 9;284(41):27857-27865. doi: 10.1074/jbc.M109.000950. Epub 2009 Aug 18. J Biol Chem. 2009. PMID: 19690169 Free PMC article.
-
Dynamic and selective nucleosome repositioning during endotoxin tolerance.J Biol Chem. 2010 Jan 8;285(2):1259-71. doi: 10.1074/jbc.M109.067330. Epub 2009 Nov 9. J Biol Chem. 2010. PMID: 19901031 Free PMC article.
-
NAD+-dependent SIRT1 deacetylase participates in epigenetic reprogramming during endotoxin tolerance.J Biol Chem. 2011 Mar 18;286(11):9856-64. doi: 10.1074/jbc.M110.196790. Epub 2011 Jan 18. J Biol Chem. 2011. PMID: 21245135 Free PMC article.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
Epigenetic and metabolic programming of innate immunity in sepsis.Innate Immun. 2019 Jul;25(5):267-279. doi: 10.1177/1753425919842320. Epub 2019 Apr 18. Innate Immun. 2019. PMID: 30997862 Free PMC article. Review.
-
New insights into the cell- and tissue-specificity of glucocorticoid actions.Cell Mol Immunol. 2021 Feb;18(2):269-278. doi: 10.1038/s41423-020-00526-2. Epub 2020 Aug 31. Cell Mol Immunol. 2021. PMID: 32868909 Free PMC article. Review.
-
Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells.J Exp Med. 2017 Dec 4;214(12):3531-3541. doi: 10.1084/jem.20171048. Epub 2017 Nov 15. J Exp Med. 2017. PMID: 29141867 Free PMC article.
-
Citrullination of histone H3 interferes with HP1-mediated transcriptional repression.PLoS Genet. 2012 Sep;8(9):e1002934. doi: 10.1371/journal.pgen.1002934. Epub 2012 Sep 13. PLoS Genet. 2012. PMID: 23028349 Free PMC article.
-
Innate immune programing by endotoxin and its pathological consequences.Front Immunol. 2015 Jan 6;5:680. doi: 10.3389/fimmu.2014.00680. eCollection 2014. Front Immunol. 2015. PMID: 25610440 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

