Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants
- PMID: 21078904
- PMCID: PMC4785892
- DOI: 10.4049/jimmunol.1002390
Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants
Abstract
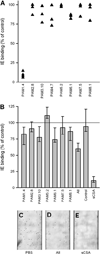
Acquired protection from Plasmodium falciparum placental malaria, a major cause of maternal, fetal, and infant morbidity, is mediated by IgG specific for the P. falciparum erythrocyte membrane protein 1 variant VAR2CSA. This protein enables adhesion of P. falciparum-infected erythrocytes to chondroitin sulfate A in the intervillous space. Although interclonal variation of the var2csa gene is lower than that among var genes in general, VAR2CSA-specific Abs appear to target mainly polymorphic epitopes. This has raised doubts about the feasibility of VAR2CSA-based vaccines. We used eight human monoclonal IgG Abs from affinity-matured memory B cells of P. falciparum-exposed women to study interclonal variation and functional importance of Ab epitopes among placental and peripheral parasites from East and West Africa. Most placental P. falciparum isolates were labeled by several mAbs, whereas peripheral isolates from children were essentially nonreactive. The mAb reactivity of peripheral isolates from pregnant women indicated that some were placental, whereas others had alternative sequestration foci. Most of the mAbs were comparable in their reactivity with bound infected erythrocytes (IEs) and recombinant VAR2CSA and interfered with IE and/or VAR2CSA binding to chondroitin sulfate A. Pair-wise mAb combinations were more inhibitory than single mAbs, and all of the mAbs together was the most efficient combination. Each mAb could opsonize IEs for phagocytosis, and a combination of the eight mAbs caused phagocytosis similar to that of plasma IgG-opsonized IEs. We conclude that functionally important Ab epitopes are shared by the majority of polymorphic VAR2CSA variants, which supports the feasibility of VAR2CSA-based vaccines against placental malaria.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




References
-
- Rogerson SJ, Hviid L, Duffy PE, Leke RFG, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect. Dis. 2007;7:105–117. - PubMed
-
- Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003;49:179–191. - PubMed
-
- Tuikue Ndam NG, Salanti A, Bertin G, Dahlbäck M, Fievet N, Turner L, Gaye A, Theander TG, Deloron P. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. J. Infect. Dis. 2005;192:331–335. - PubMed
-
- Magistrado P, Salanti A, Tuikue Ndam NG, Mwakalinga SB, Resende M, Dahlbäck M, Hviid L, Lusingu J, Theander TG, Nielsen MA. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J. Infect. Dis. 2008;198:1071–1074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

