Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas
- PMID: 21078963
- PMCID: PMC3000297
- DOI: 10.1073/pnas.1012525107
Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas
Abstract
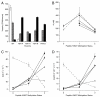
EZH2, the catalytic subunit of the PRC2 complex, catalyzes the mono- through trimethylation of lysine 27 on histone H3 (H3K27). Histone H3K27 trimethylation is a mechanism for suppressing transcription of specific genes that are proximal to the site of histone modification. Point mutations of the EZH2 gene (Tyr641) have been reported to be linked to subsets of human B-cell lymphoma. The mutant allele is always found associated with a wild-type allele (heterozygous) in disease cells, and the mutations were reported to ablate the enzymatic activity of the PRC2 complex for methylating an unmodified peptide substrate. Here we demonstrate that the WT enzyme displays greatest catalytic efficiency (k(cat)/K) for the zero to monomethylation reaction of H3K27 and diminished efficiency for subsequent (mono- to di- and di- to trimethylation) reactions. In stark contrast, the disease-associated Y641 mutations display very limited ability to perform the first methylation reaction, but have enhanced catalytic efficiency for the subsequent reactions, relative to the WT enzyme. These results imply that the malignant phenotype of disease requires the combined activities of a H3K27 monomethylating enzyme (PRC2 containing WT EZH2 or EZH1) together with the mutant PRC2s for augmented conversion of H3K27 to the trimethylated form. To our knowledge, this is the first example of a human disease that is dependent on the coordinated activities of normal and disease-associated mutant enzymatic function.
Conflict of interest statement
Conflict of interest statement: All authors are employees and stockholders of Epizyme, Inc.
Figures
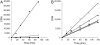


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

