Specific posttranslational modification regulates early events in mammary carcinoma formation
- PMID: 21078982
- PMCID: PMC3000280
- DOI: 10.1073/pnas.1013405107
Specific posttranslational modification regulates early events in mammary carcinoma formation
Abstract
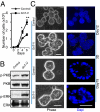
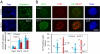
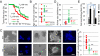
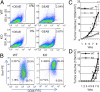
The expression of an enzyme, GnT-V, that catalyzes a specific posttranslational modification of a family of glycoproteins, namely a branched N-glycan, is transcriptionally up-regulated during breast carcinoma oncogenesis. To determine the molecular basis of how early events in breast carcinoma formation are regulated by GnT-V, we studied both the early stages of mammary tumor formation by using 3D cell culture and a her-2 transgenic mouse mammary tumor model. Overexpression of GnT-V in MCF-10A mammary epithelial cells in 3D culture disrupted acinar morphogenesis with impaired hollow lumen formation, an early characteristic of mammary neoplastic transformation. The disrupted acinar morphogenesis of mammary tumor cells in 3D culture caused by her-2 expression was reversed in tumors that lacked GnT-V expression. Moreover, her-2-induced mammary tumor onset was significantly delayed in the GnT-V null tumors, evidence that the lack of the posttranslational modification catalyzed by GnT-V attenuated tumor formation. Inhibited activation of both PKB and ERK signaling pathways was observed in GnT-V null tumor cells. The proportion of tumor-initiating cells (TICs) in the mammary tumors from GnT-V null mice was significantly reduced compared with controls, and GnT-V null TICs displayed a reduced ability to form secondary tumors in NOD/SCID mice. These results demonstrate that GnT-V expression and its branched glycan products effectively modulate her-2-mediated signaling pathways that, in turn, regulate the relative proportion of tumor initiating cells and the latency of her-2-driven tumor onset.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ménard S, et al. HER2 overexpression in various tumor types, focussing on its relationship to the development of invasive breast cancer. Ann Oncol. 2001;12(Suppl 1):S15–S19. - PubMed
-
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. - PubMed
-
- Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

