Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia
- PMID: 21078987
- PMCID: PMC3000270
- DOI: 10.1073/pnas.1011949107
Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia
Abstract
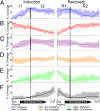
The mechanism(s) by which anesthetics reversibly suppress consciousness are incompletely understood. Previous functional imaging studies demonstrated dynamic changes in thalamic and cortical metabolic activity, as well as the maintained presence of metabolically defined functional networks despite the loss of consciousness. However, the invasive electrophysiology associated with these observations has yet to be studied. By recording electrical activity directly from the cortical surface, electrocorticography (ECoG) provides a powerful method to integrate spatial, temporal, and spectral features of cortical electrophysiology not possible with noninvasive approaches. In this study, we report a unique comprehensive recording of invasive human cortical physiology during both induction and emergence from propofol anesthesia. Propofol-induced transitions in and out of consciousness (defined here as responsiveness) were characterized by maintained large-scale functional networks defined by correlated fluctuations of the slow cortical potential (<0.5 Hz) over the somatomotor cortex, present even in the deeply anesthetized state of burst suppression. Similarly, phase-power coupling between θ- and γ-range frequencies persisted throughout the induction and emergence from anesthesia. Superimposed on this preserved functional architecture were alterations in frequency band power, variance, covariance, and phase-power interactions that were distinct to different frequency ranges and occurred in separable phases. These data support that dynamic alterations in cortical and thalamocortical circuit activity occur in the context of a larger stable architecture that is maintained despite anesthetic-induced alterations in consciousness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. - PubMed
-
- Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: Brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn. 2000;9:370–386. - PubMed
-
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

